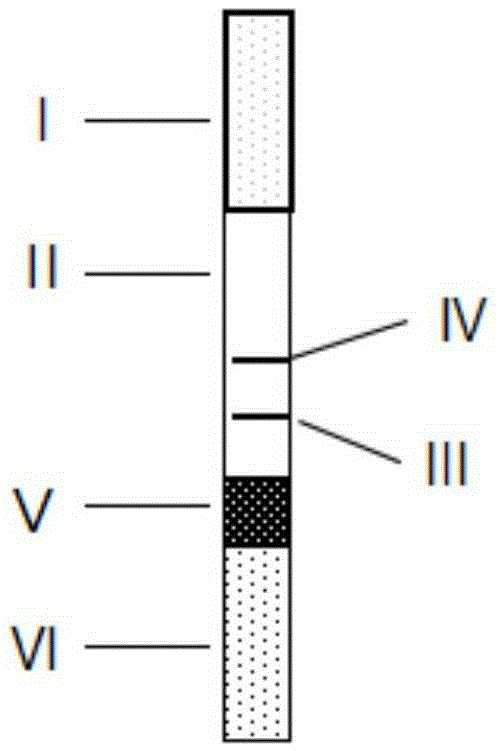
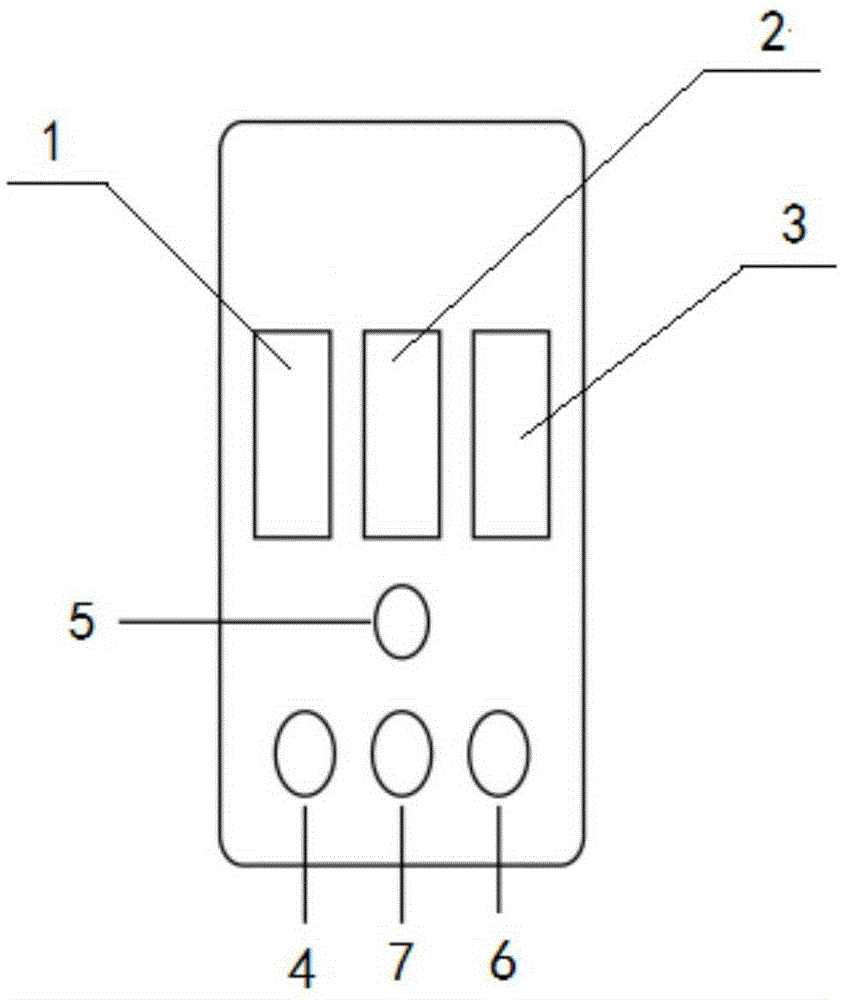
Detection device for simultaneously and quantitatively detecting SAA/PCT/CRP
A quantitative detection and detection device technology, applied in the field of medical detection, can solve problems such as increased CRP content, and achieve the effects of low cost, high detection sensitivity, and simple and stable detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1 main material
[0049] 1.1 Biological materials SAA, PCT and CRP paired antibodies, purchased from Finland HYTEST company; goat anti-mouse IgG: homemade; chloroauric acid: Sigma company product; NC membrane: Sartorius company product; bovine serum albumin (BSA), polyethylene Alcohol PEG20000: Sigma product. Other commonly used reagents are analytical reagents.
[0050] 1.2 Clinical samples were obtained by the company in related hospitals, a total of 120 samples, of which 60 were serum samples of patients diagnosed with inflammatory infection, the SAA detection value was 5-200mg / L, the PCT detection value was 0.5-20mg / L and 1-200mg / L. In 60 normal human serum samples, the SAA, PCT and CRP values were not detected.
[0051] 1.3 Triple test card: designed by our company, and produced and provided by related companies as required.
[0052] 1.4 Immune Chromatography Results Interpretation Recorder: Model: NS3001, a product of Tianjin Zhongxin Keju Biopharmaceutical Co., Ltd...
Embodiment 2
[0067] A test strip for simultaneous rapid and quantitative detection of SAA / PCT / CRP is prepared by the following method. The specific steps are:
[0068] (1) SAA, PCT and CRP antibody colloidal gold labeling. Prepare a colloidal gold solution with a diameter of 40±5nm by the chloroauric acid-trisodium citrate method. Take three colloidal gold solutions and adjust the solution to SAA with 0.2MK2CO3. Adjust the pH of PCT to 7.5, the pH of PCT to 6.5 and the pH of CRP to 8.0, and then slowly stir the colloidal gold. SAA: add 10ug per ml of solution to add SAA-labeled antibody to the solution; PCT: per ml of solution Add 15ug to add PCT-labeled antibody to the solution; CRP: add 5ug per ml of solution to add CRP-labeled antibody to the solution, continue to stir for 30 minutes, and then add to the final concentration of 0.5% PEG2000 and 0.5% bovine serum albumin (BSA) for blocking, after labeling, centrifuge at 13000r / min, discard the supernatant, and reconstitute the precipitate in...
Embodiment 3
[0072] (1) SAA, PCT and CRP antibody colloidal gold labeling. Prepare a colloidal gold solution with a diameter of 40±5nm by the chloroauric acid-trisodium citrate method. Take three colloidal gold solutions and adjust the solution to SAA with 0.2MK2CO3. Adjust the pH of PCT to 7.0, the pH of PCT to 7.0 and the pH of CRP to 7.0, and then slowly stir the colloidal gold. SAA: Add 8ug per ml of solution to add SAA-labeled antibody to the solution; PCT: per ml of solution Add 18ug to add PCT-labeled antibody to the solution; CRP: add 8ug per ml of solution to add CRP-labeled antibody to the solution, continue to stir for 30 minutes, and then add to the final concentration of 0.5% PEG2000 and 0.5% bovine serum albumin (BSA) for blocking, after labeling, centrifuge at 13000r / min, discard the supernatant, and reconstitute the precipitate into colloidal gold working solutions of different proportions (0.1MTris.HCl buffer, pH7.8, containing 70% of the original volume) 0.2% bovine serum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com