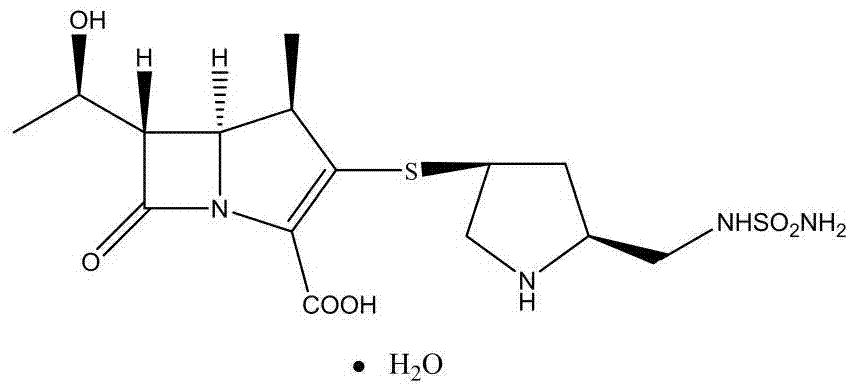
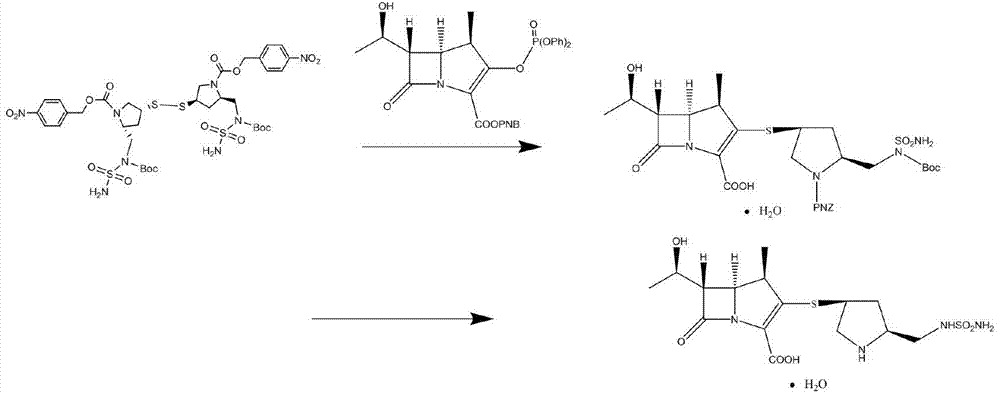
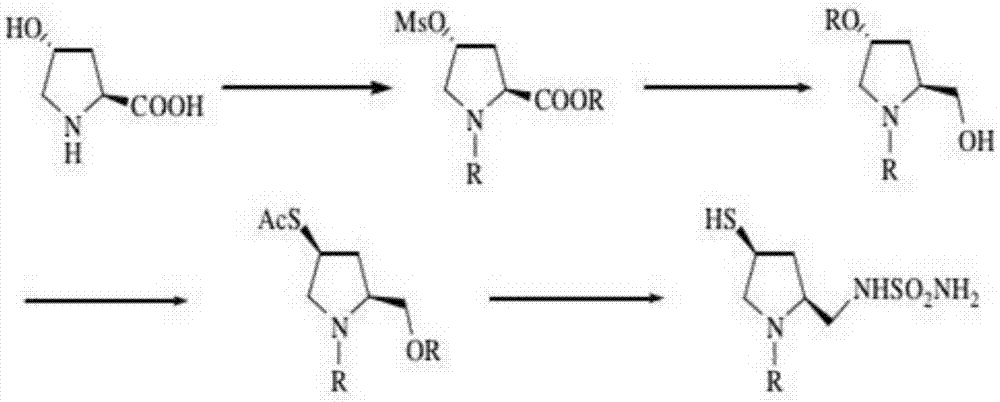
Preparation method and application of doripenem side chain disulfide
A technology of disulfide and disulfide, applied in the field of preparation of doripenem side chain disulfide, can solve problems such as unfavorable purification of doripenem, and achieve the effects of process optimization and convenient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A preparation method of doripenem side chain disulfide, comprising the following steps:
[0045] 1) Dissolving 10 grams of doripenem side chains in 40 grams of methanol to form a methanol solution of A; adding 3 grams of 30% sodium methoxide-methanol solution to the resulting methanol solution of doripenem side chains, The dropping rate was 0.3 g / min, mechanically stirred at a speed of 100 rpm, and reacted at 20° C. for 1.2 hours to obtain a pale yellow clear liquid, which was sampled for HPLC to detect the reaction;
[0046] 2) Dissolve 2 grams of ferric chloride in 8 grams of methanol to obtain a suspension, add the resulting suspension to the light yellow clear liquid obtained in step 1), and stir mechanically at a temperature of 20°C at a speed of 100 rpm to form a mixed solution;
[0047] 3) Introduce oxygen into the mixed liquid, keep the oxygen pressure at 0.08MPa, react at 20°C for 3 to 4 hours, and the HPLC test shows that the reaction is complete, and the rea...
Embodiment 2
[0052] A preparation method of doripenem side chain disulfide, comprising the following steps:
[0053] 1) Dissolving 20 grams of doripenem side chains in 40 grams of methanol to form a methanol solution of A; adding 3.5 grams of 30% sodium methoxide-methanol solution to the resulting methanol solution of doripenem side chains, The rate of addition was 0.4 g / min, and the speed was 300 r / min with mechanical stirring, and reacted at 30° C. for 1.5 hours to obtain a light yellow clear liquid, which was sampled for HPLC to detect the reaction;
[0054] 2) Dissolve 5 grams of ferric chloride in 10 grams of methanol to obtain a suspension, add the resulting suspension to the light yellow clear liquid obtained in step 1), and stir mechanically at a temperature of 30°C at a speed of 100 rpm to form a mixed solution;
[0055] 3) Introduce oxygen into the mixed liquid, keep the oxygen pressure at 0.12MPa, react at 30°C for 2.5h, and the HPLC test shows that the reaction is complete, an...
Embodiment 3
[0060] A preparation method of doripenem side chain disulfide, comprising the following steps:
[0061] 1) Dissolving 30 grams of doripenem side chains in 180 grams of methanol to form a methanol solution of A; adding 3.2 grams of 30% sodium methoxide-methanol solution to the resulting methanol solution of doripenem side chains, The rate of addition was 0.35 g / min, and the speed was 200 r / min with mechanical stirring, and reacted at 25°C for 1.3 hours to obtain a light yellow clear liquid, which was sampled for HPLC to detect the reaction;
[0062] 2) Dissolve 7.5 grams of ferric chloride in 18 grams of methanol to obtain a suspension, which is added to the light yellow clear liquid obtained in step 1), and mechanically stirred at a temperature of 28°C at a speed of 200 rpm to form a mixed solution;
[0063] 3) Introduce oxygen into the mixed liquid, keep the oxygen pressure at 0.10 MPa, react at 25°C for 2.8 hours, and the reaction is complete as detected by HPLC, and the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com