Method for producing lead sulfate with electrochemical activity from waste lead-acid batteries
A lead-acid battery and lead sulfate technology, applied in the direction of lead sulfate, etc., can solve problems such as sales difficulties, low prices, and output reduction, and achieve the effects of reducing production costs, simple process, and reducing pollution risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
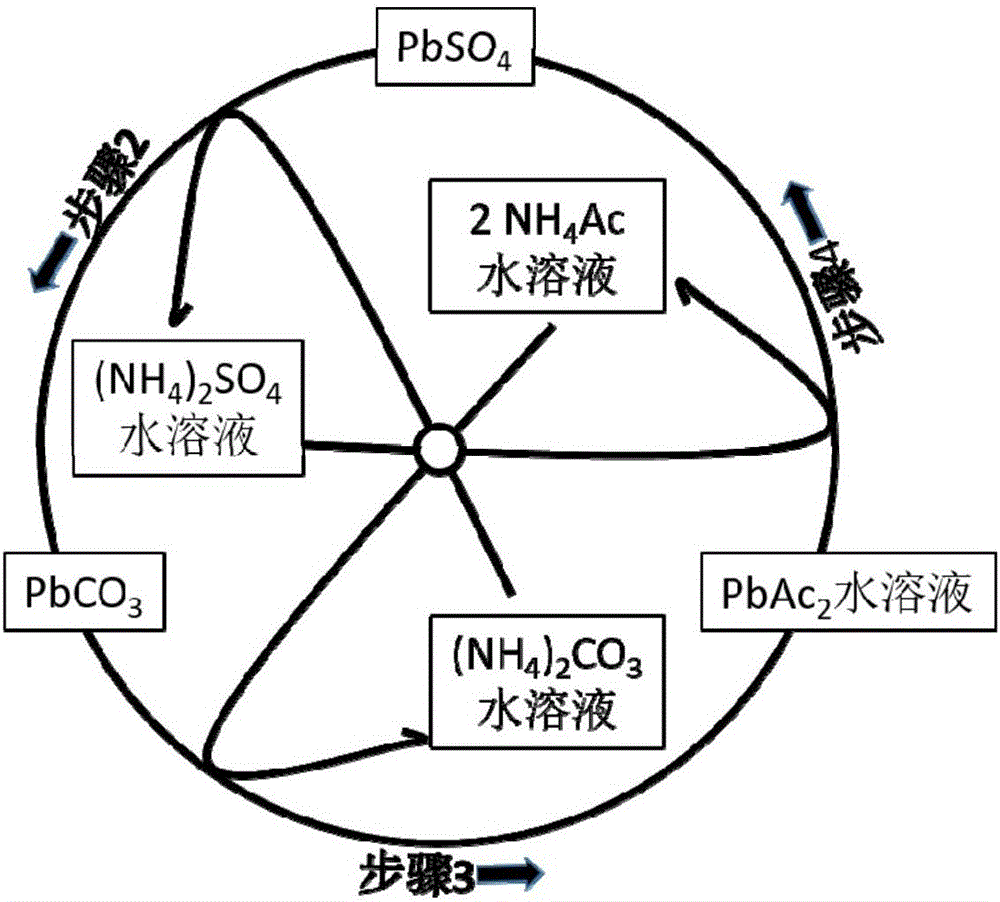
[0052] Step (1): Gently disassemble the lead-acid battery to obtain the positive electrode sheet and the negative electrode sheet, and then separate the lead alloy grid and the electrode powder to obtain the positive electrode grid alloy, the negative electrode grid alloy, the positive electrode powder and the negative electrode powder. The content of lead sulfate in positive electrode powder and negative electrode powder was analyzed respectively.
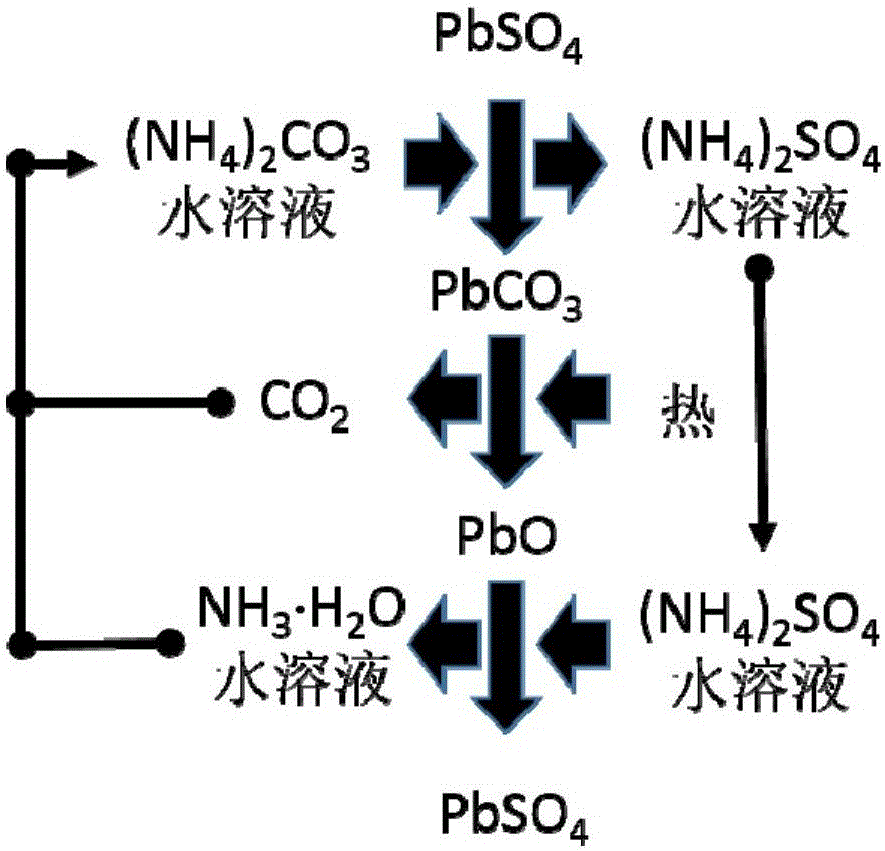
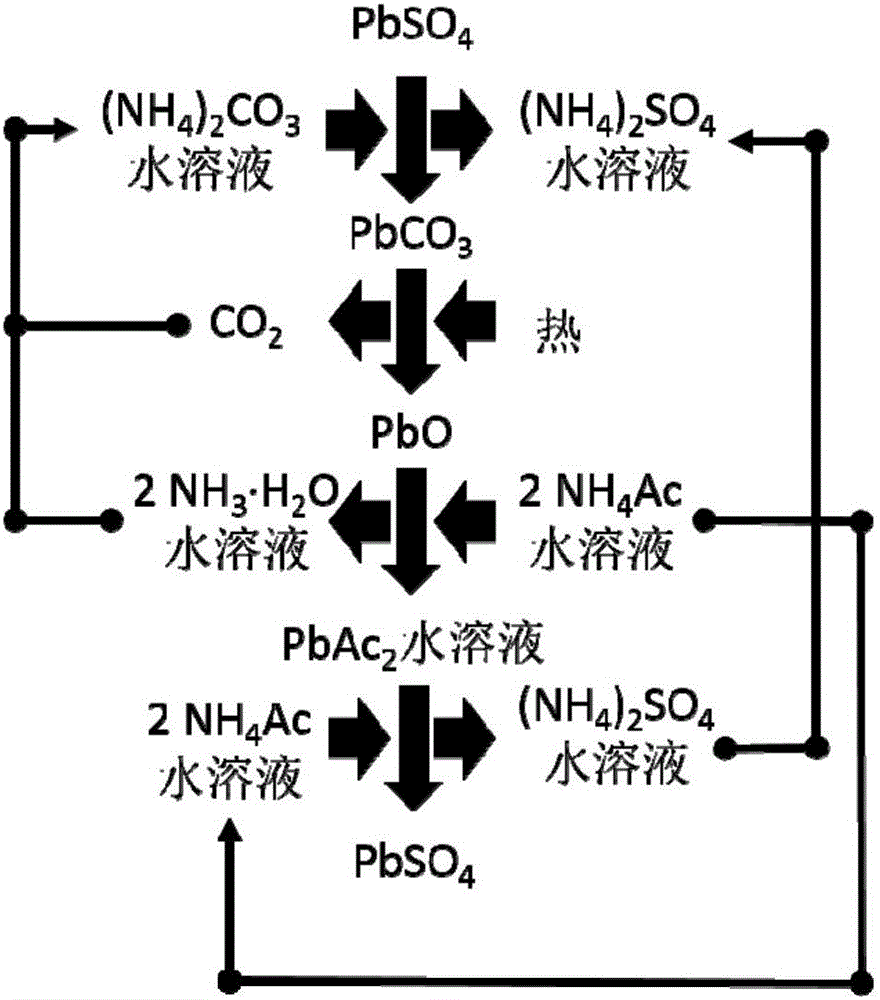
[0053] Step (2): Put the obtained positive electrode powder (or negative electrode powder) into a stirred reactor, and then press the PbSO 4 Add sufficient amount of ammonium carbonate solution and stir to make it react. The reaction temperature is between room temperature and 50°C, and the reaction time is between 0.5 and 3 hours. Filter or centrifuge the solid (for positive electrode powder, the solid is PbO 2 and PbCO 3 The mixture of the negative electrode powder is Pb and PbCO 3 mixture) and solution (ammonium sulfate sol...
Embodiment 2
[0060] Step (1): Gently disassemble the lead-acid battery to obtain the positive electrode sheet and the negative electrode sheet, and then separate the lead alloy grid and the electrode powder to obtain the positive electrode grid alloy, the negative electrode grid alloy, the positive electrode powder and the negative electrode powder. The content of lead sulfate in positive electrode powder and negative electrode powder was analyzed respectively.
[0061] Step (2): Put the obtained positive electrode powder (or negative electrode powder) into a stirred reactor, and then press the PbSO 4 Add sufficient amount of ammonium carbonate solution and stir to make it react. The reaction temperature is between room temperature and 50°C, and the reaction time is between 0.5 and 3 hours. Filter or centrifuge the solid (for positive electrode powder, the solid is PbO 2 and PbCO 3 The mixture of the negative electrode powder is Pb and PbCO 3 mixture) and solution (ammonium sulfate sol...
Embodiment 3
[0068] Step (1): Gently disassemble the lead-acid battery to obtain the positive electrode sheet and the negative electrode sheet, and then separate the lead alloy grid and the electrode powder to obtain the positive electrode grid alloy, the negative electrode grid alloy, the positive electrode powder and the negative electrode powder. The content of lead sulfate in positive electrode powder and negative electrode powder was analyzed respectively.
[0069] Step (2): Put the obtained positive electrode powder (or negative electrode powder) into a stirred reactor, and then press the PbSO 4 Add sufficient amount of ammonium carbonate solution and stir to make it react. The reaction temperature is between room temperature and 50°C, and the reaction time is between 0.5 and 3 hours. Filter or centrifuge the solid (for positive electrode powder, the solid is PbO 2 and PbCO 3 The mixture of the negative electrode powder is Pb and PbCO 3 mixture) and solution (ammonium sulfate sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com