Hydrobromide paroxetine-sec-butyl alcohol compound and preparation method thereof
A technology of alcoholate and hydrobromic acid, applied in the direction of organic chemistry, etc., can solve the problems of high desolvation temperature, large particle size, low purity of vortioxetine hydrobromide α crystal form, etc., and achieve desolvation Low melting temperature, high crystal purity, suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
[0044] The preparation of embodiment 1-sec-butanol compound
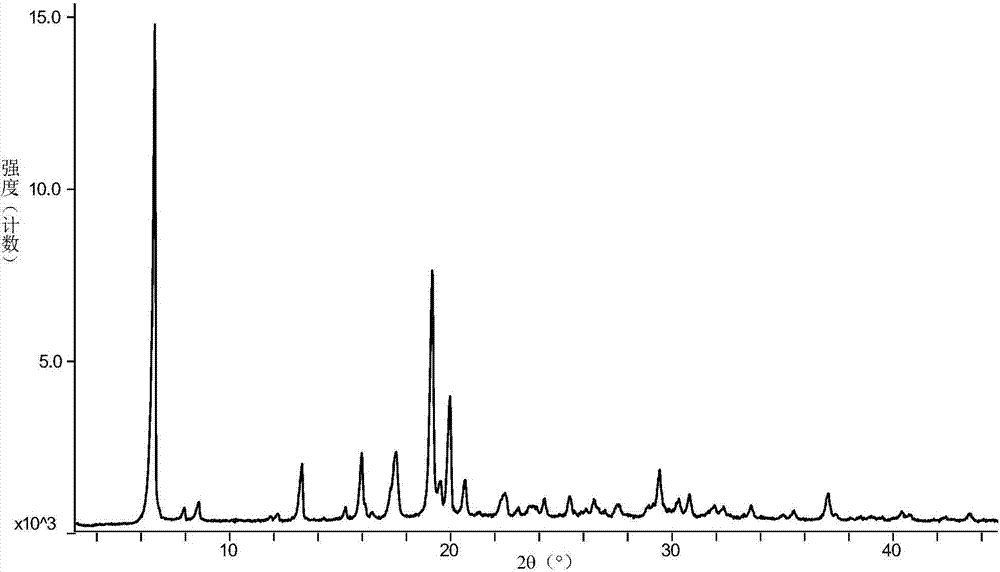
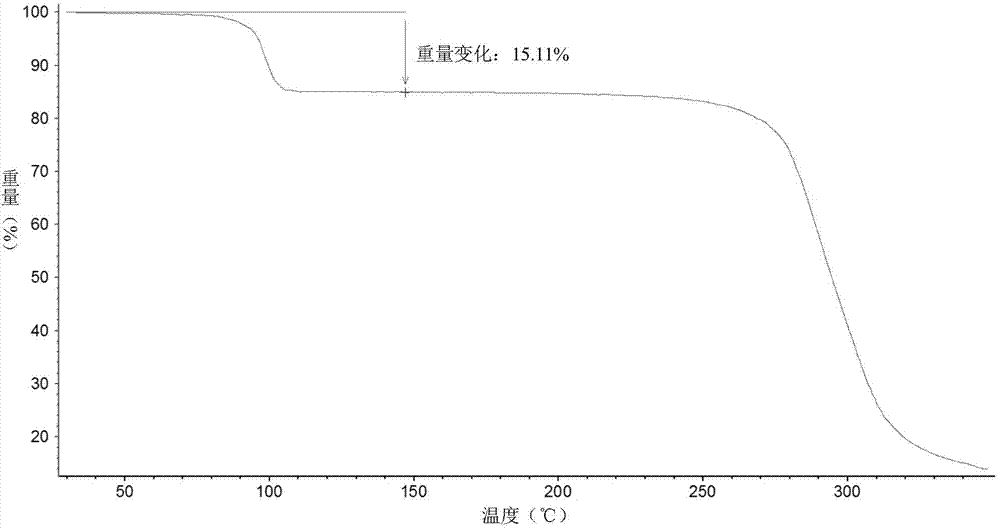
[0045] Add 29g of vortioxetine hydrobromide and 524ml of sec-butanol into a 1L reaction flask, heat to 100°C and reflux for 30min, then lower the temperature at a rate of 0.2°C / min until it is cooled to room temperature, filter, and dry at 30°C for 2h. 27.2 g of white solid was obtained. Its X-diffraction, TGA, DSC atlas see Figure 1~3 , and its X-ray powder diffraction data are shown in Table 2.
[0046] X-ray powder diffraction expressed in 2θ angles figure 1 There are characteristic peaks at 6.61±0.2, 13.26±0.2, 15.98±0.2, 17.47±0.2, 19.17±0.2, 19.97±0.2 and 20.64±0.2.
[0047] its TGA figure 2 The thermal weight loss is about 15.11%, which is close to one molecule of sec-butanol, so it is a sec-butanol compound.
[0048] Its DSC image 3 Among them, there are endothermic peaks at 60.2°C, 103.0°C and 226.2°C.
Embodiment 2 1
[0049] The preparation of embodiment 2-sec-butanol compound
[0050] Heat 5 g of vortioxetine hydrobromide and 100 ml of sec-butanol to 100°C and reflux for 30 minutes, then lower the temperature at a rate of 0.5°C / min until cooled to room temperature, filter, and dry at 20°C for 2 hours to obtain 4.8 g of white solid .
Embodiment 3 1
[0051] The preparation of embodiment 3-sec-butanol compound
[0052] Heat 5 g of vortioxetine hydrobromide and 100 ml of sec-butanol to 100°C and reflux for 30 minutes, then lower the temperature at a rate of 0.1°C / min until it cools to room temperature, filter, and dry at 40°C for 2 hours to obtain 4.5 g of white solid .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com