Human parainfluenza virus I, II, III, and IV-type quadruple-PCR detection kit, and detection method thereof
A human parainfluenza virus, detection kit technology, applied in biochemical equipment and methods, recombinant DNA technology, microbial determination/inspection, etc. Simple and effective for improving amplification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
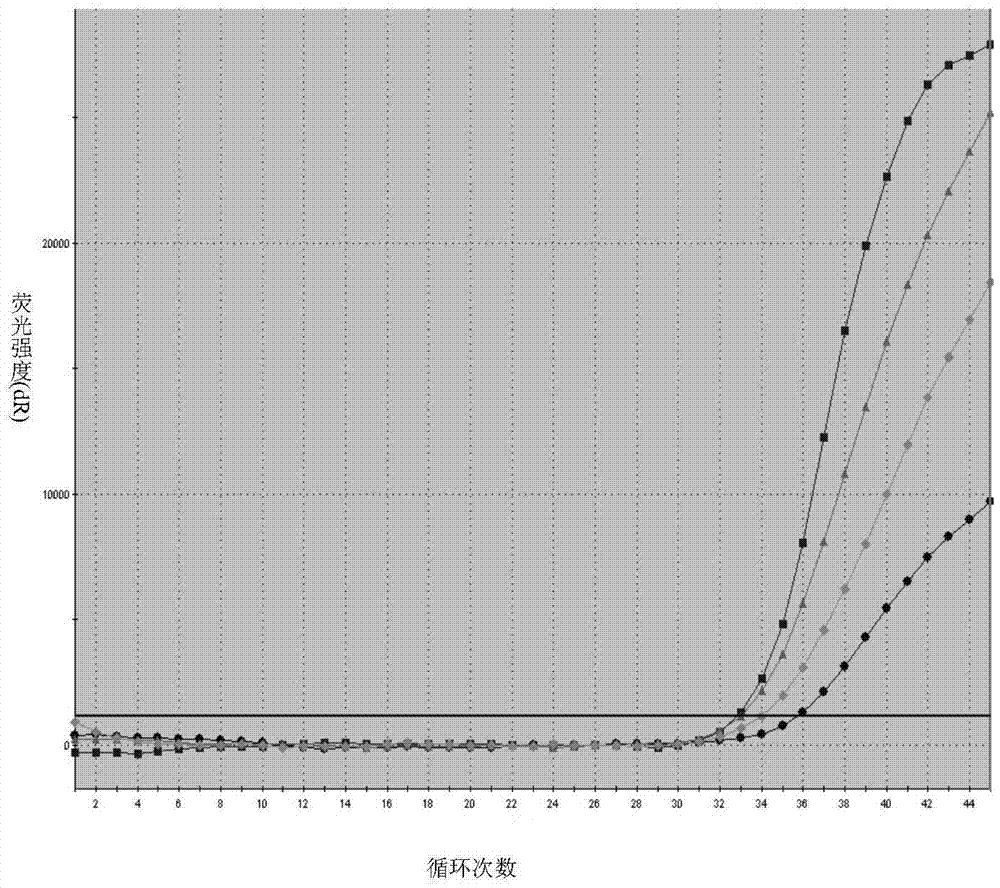
[0096] A quadruple PCR detection kit for human parainfluenza virus types I, II, III, and IV, including a PCR reaction solution, a negative control and a positive control, wherein the specification of the PCR reaction solution is: 8 tubes×6 strips, 20 μl / tube, and the components are shown in Table 1 below; the positive control specification is 1 tube, 40 μl / tube, and the components are RNA transcribed in vitro after the synthetic sequence of the detection region of human parainfluenza virus type I, II, III, and IV, using Tris-EDTA The buffer solution (0.01MpH8.0) was diluted and stored frozen; the specification of the negative control was 1 tube, 40 μl / tube, and the composition was Tris-EDTA buffer solution ( 0.01M pH8.0).
[0097] Table 1
[0098]
[0099]
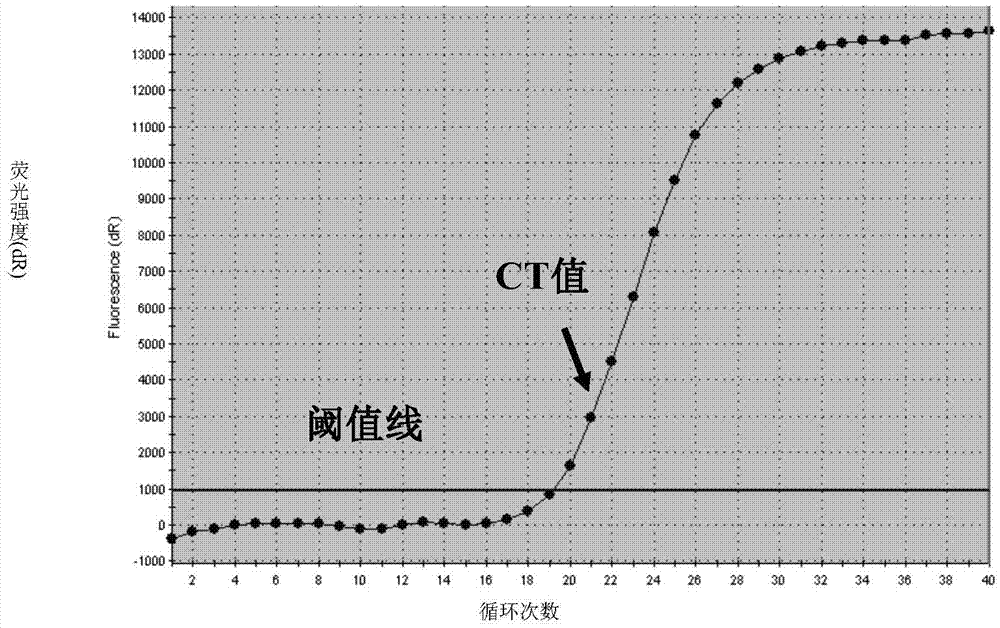
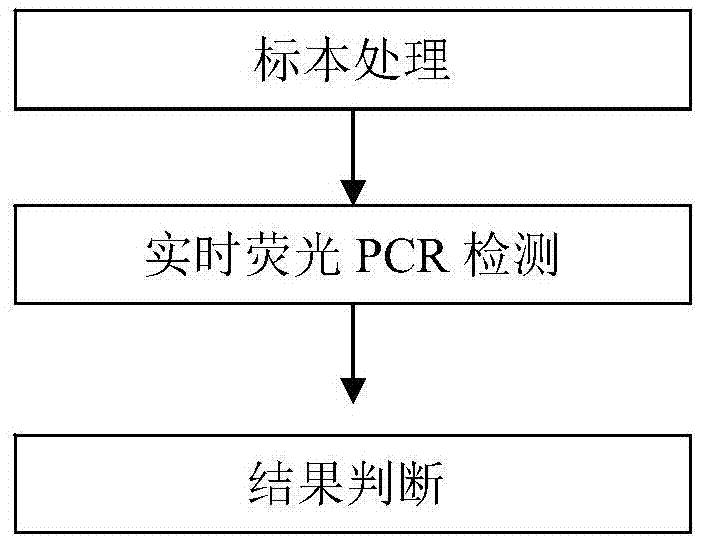
[0100] A quadruple PCR detection method for human parainfluenza virus types I, II, III, and IV. The method uses the above-mentioned quadruple PCR detection kit for human parainfluenza virus types I, II, III, and IV...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com