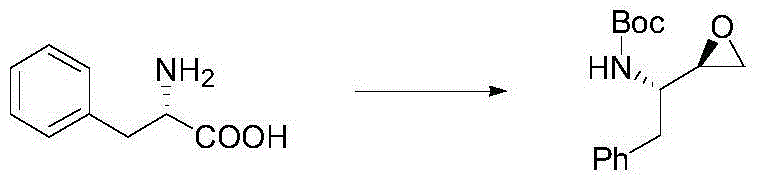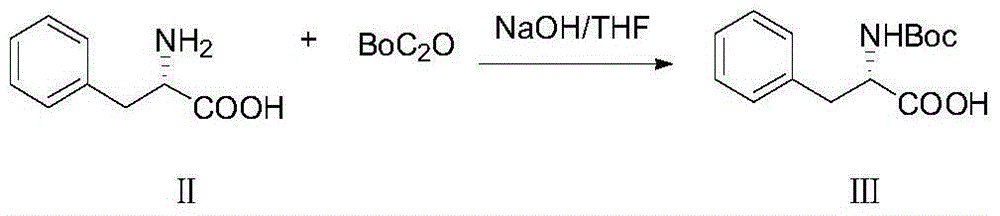Synthetic method for chiral epoxy compound of anti-HIV drug intermediate
A technology of epoxy compound and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of complex process and high cost, and achieve the effects of simple and easy synthesis method, reduced synthesis cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of BOC-L-phenylalanine (compound of formula III)
[0023]
[0024] Add 1060mL of water into a 3L three-necked flask, add 106g of NaOH solid under ice bath, and stir until the temperature Tmax=52°C. When the temperature drops below 30°C, add 198.4g of L-phenylalanine (compound of formula II), Add THF1056mL, when T=22℃, add BoC dropwise 2 O289g, titrated for 22 minutes, at this time, the internal temperature was 37°C, reacted overnight at room temperature, and the system produced a large amount of white solid;
[0025] TLC was sampled the next day, the raw materials had been reacted, THF was removed under reduced pressure (45° C.), and the remaining aqueous phase was milky white. HCL was added thereto to adjust the pH=1, and CH 2 CL 2 (1+1) extraction twice, no product in the TLC aqueous phase, the organic phase was washed once with 500 mL of brine, and dried over sodium sulfate. The organic phase was spin-dried to obtain 332 g of an oily l...
Embodiment 2
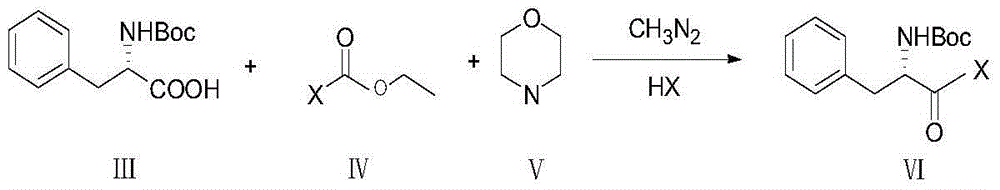
[0026] Embodiment 2: prepare the chloroketone compound (a kind of formula VI compound) of Boc-L-phenylalanine
[0027]
[0028] Add 66.3 g of the compound of formula III and 500 mL of THF into a 1 L three-necked flask, cool down to -25° C., dropwise add 29.15 g of the compound of formula IV into the three-necked flask, and stir for 30 minutes. 27.18 g of the compound of formula V was added dropwise, reacted for 92 minutes, and kept at -25 to -28°C. A large amount of white solids were produced during the dropwise addition. Quickly filter at -5°C, wash the filter cake twice with THF (50+50) mL, transfer the filtrate to a 2L three-neck flask, cool down at about 0°C, replace with nitrogen three times, and add CH to the system dropwise 3 N 2 370mL, reacted for 90 minutes, the temperature was maintained at about 0°C, the system changed from colorless to yellow-green, and 400mL of HCl was added to the system to react overnight (room temperature);
[0029] The next day, separate ...
Embodiment 3
[0031] Embodiment 3: prepare the chloroketone compound (a kind of formula VI compound) of Boc-L-phenylalanine
[0032]
[0033] Add 70.4g of compound of formula III and 530mL of THF into a 1L three-necked flask, cool down to below -28°C, add 30g of compound of formula IV dropwise into the three-necked flask, stir for 30 minutes after dropping, add 27.5g of compound of formula V dropwise, and react for 85 minutes. Heat the system from -28°C to -30°C to produce a large amount of white solids, quickly filter at -5°C, transfer the filtrate to a 2L three-necked flask, protect the system with nitrogen, and add CH to the system dropwise 3 N 2 400mL, react for 50 minutes, maintain the temperature at around 0°C, the system turns yellow, add 700mL of HCl dropwise to the system after 1 hour, and react overnight at room temperature;
[0034] Separate the liquid the next day, collect the organic phase, use MTBE (200+200) mL for the aqueous phase until there is no product on the TLC of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com