Application of a compound in the preparation of rtki and/or anti-angiogenic drugs
An anti-angiogenesis and angiogenesis technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems that the use of tyrosine kinase has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
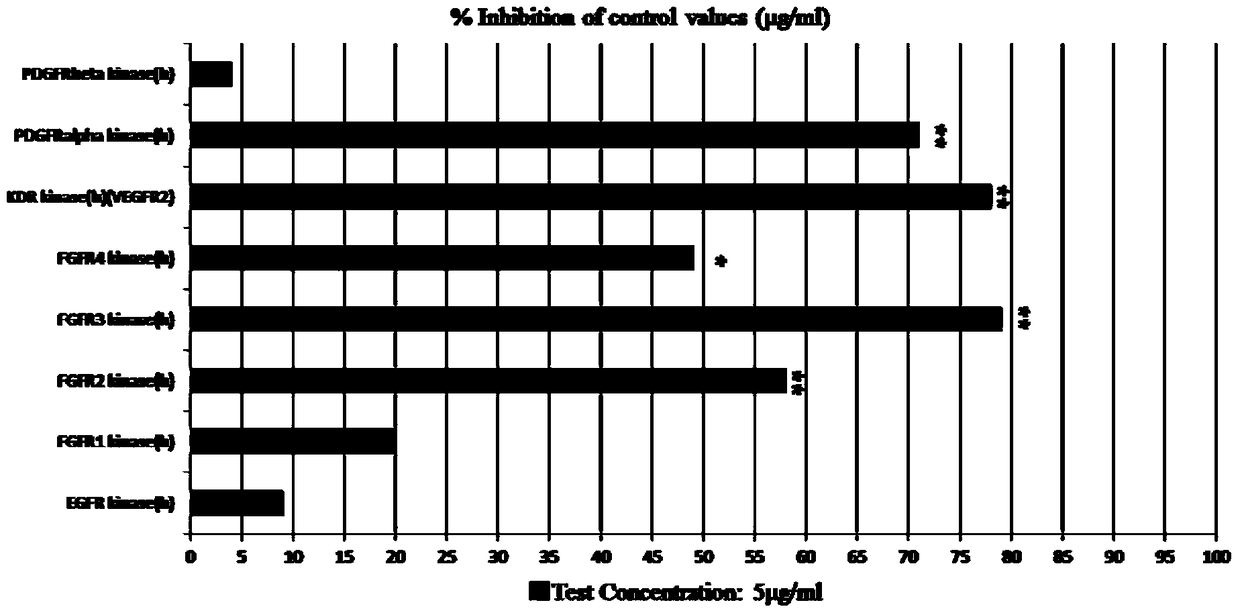
[0020] Bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane kinase activity study:
[0021] 1. Experimental drugs
[0022] Bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane (for the preparation process, please refer to the patent CN101597213B previously applied by the laboratory), using DMSO to aid dissolution, configured as a 50mg / ml storage solution for long-term storage, for experimental use The concentration was 5 μg / ml.
[0023] 2. Kinase Activity Detection
[0024] The kinase domains of FGFR1, FGFR2, FGFR3, FGFR4, PDGFR-α, PDGFR-β and VEGFR2 were assayed in buffers containing the following substances. The buffer contains 50mM HEPES (pH 7.0), 2mM MgCl2, 10mM MnCl2, 1mM NaF, 1mMDTT (dithiothreitol), 1mg / mL BSA (bovine serum albumin), 0.25μM biological peptide matrix (CAGAGAIETDKEYYTVKD). Add ATP at a final concentration of 12.5 μM to 90 μl buffer, and then add 5 μl of FGFR1, FGFR2, FGFR3, FGFR4, PDGFR-α, PDGFR-β and VEGFR2 respectively, and incubate at 37°C for 1 hour for kinase r...
Embodiment 2
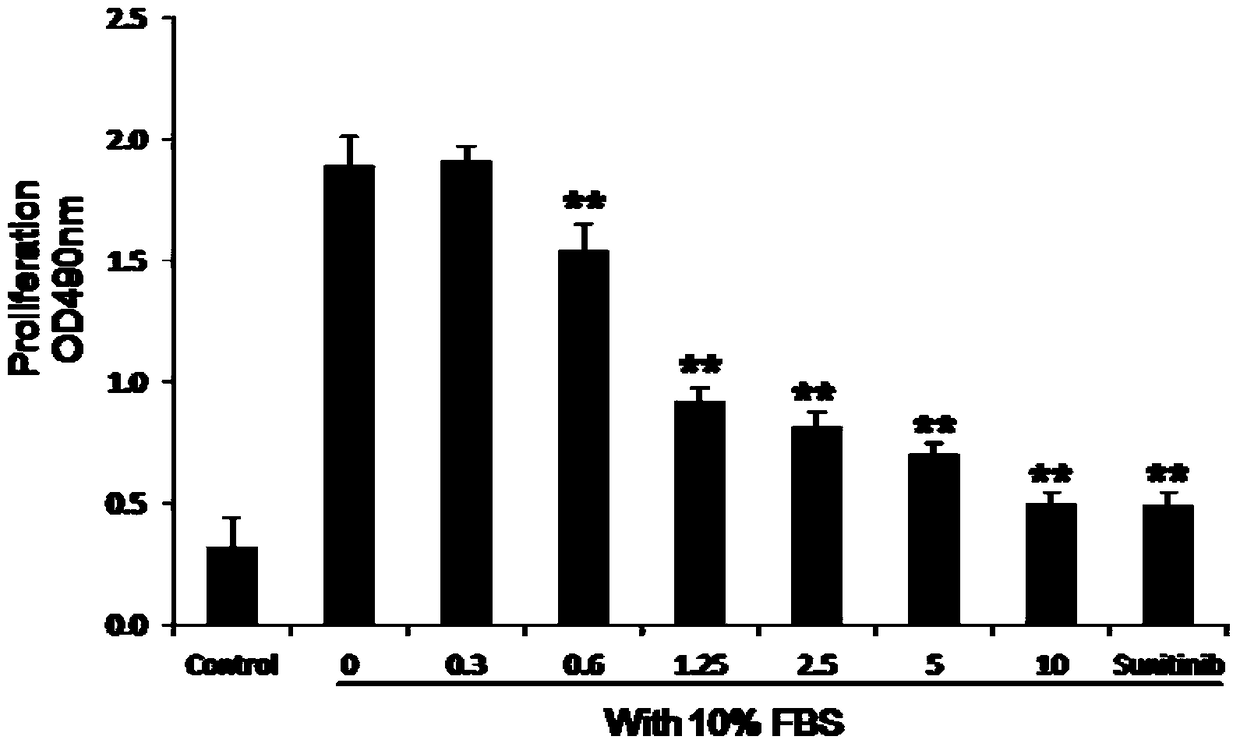
[0027] Study on the proliferation of HUVEC with bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane:
[0028] 1. Cell line
[0029] Human umbilical vein endothelial cell line (HUVEC) was purchased from Shanghai Cell Bank, Chinese Academy of Sciences. In DMEM medium containing 10% fetal bovine serum at 37°C, with a volume fraction of 5% CO 2 1. Routine culture in the air under fully saturated humidity conditions, replace the medium after 48 hours, when the cell growth reaches saturation, digest with 0.25% trypsin and passage once every 2-3 days, and use logarithmic growth phase cells in the experiment.
[0030] 2. Experimental drugs
[0031] Bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane and Sunitinib, solubilized with DMSO and diluted in DMEM medium to 0.3, 0.6, 1.25, 2.5, 5, 10 and 6.25 μg / ml.
[0032] 3. Detection of HUVEC cell proliferation
[0033] Take HUVEC cells in the logarithmic growth phase by 4×10 3 Cells were seeded per well in a 96-well culture plate. Before use,...
Embodiment 3
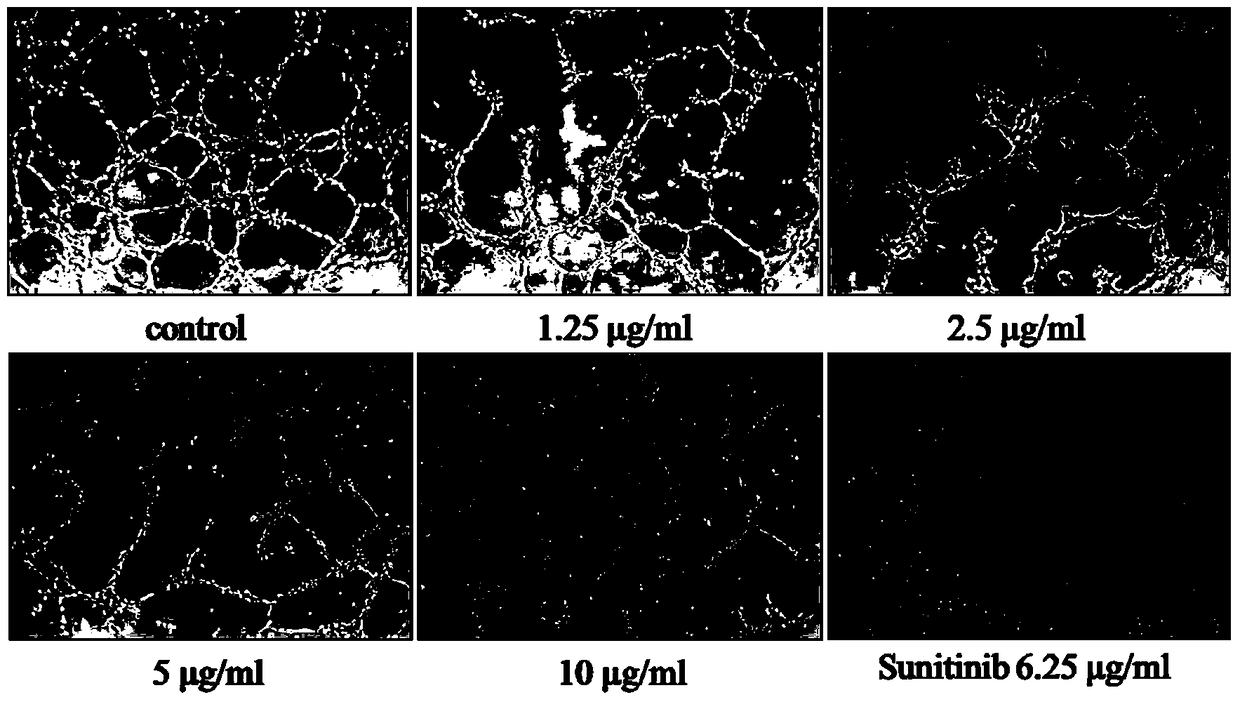
[0036] Anti-angiogenesis of HUVEC by bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane:
[0037] Materials and Methods
[0038] 1. Experimental cells:
[0039] Same as Example 2
[0040] 2. Experimental drugs:
[0041] Same as Example 2
[0042] 3. Experimental method:
[0043] Matrigel containing growth factors was diluted in collagen (1:6, volume to volume), and kept at low temperature (on ice). This mixture was added to a 96-well cell culture plate (50ul / well), and incubated at 37°C for 1 hour to fully solidify the solution.
[0044]Inoculate these wells with HUVEC cell suspensions unmixed or mixed with 1.25, 2.5, 5, 10 μg / ml bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane and 6.25 μg / ml Sunitinib , continue to cultivate for 8h, and take pictures. IPP 6.0 software was used to measure the total capillary length of each well to evaluate the effect of bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane on capillary formation.
[0045] The results showed that bis-(2,3-dibromo-4,5-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com