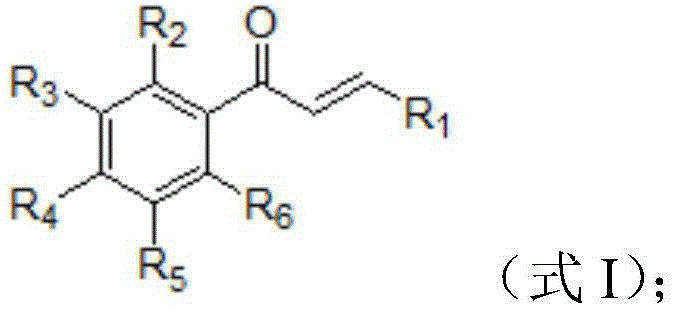
3-substituted propyl-2-alkene-1-ketone compound and application thereof
A technology of ketone compounds and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
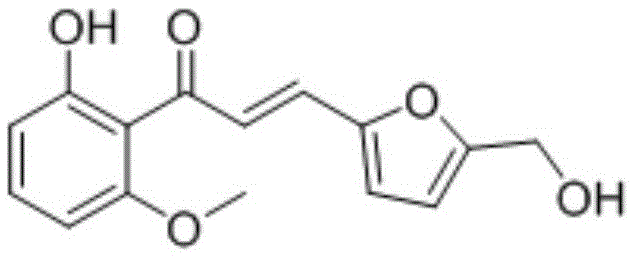
[0106] The preparation of embodiment 1 (E)-1-(2-hydroxyl-6-methoxyphenyl)-3-(5-hydroxymethylfuran-2-yl) prop-2-en-1-one (1 )
[0107]
[0108] Take 500mg (3.0mmol) of 2-hydroxy-6-methoxyacetophenone and 600mg (3.6mmol) of 5-hydroxymethylfurfural and add it to 15ml ethanol, then add 30% NaOH aqueous solution, and stir the reaction at room temperature After 24-48 hours, TLC showed that the reaction was almost complete. The reaction liquid was poured into ice water, filtered, and the solid was washed with water three times. After drying, it was separated by flash column to obtain compound (1), 312 mg, with a yield of 38%.
[0109] 1 H-NMR: 3.95 (3H, s, -OCH3), 4.69 (2H, s, -CH 2 -); 6.43(2H,m,Ar-H); 6.60(1H,dd,J 1 =8.4Hz,J 2 = 0.9Hz, Ar-H); 6.66 (1H, d, J = 3.3Hz, Ar-H); 7.38 (2H, m, Ar-H); 7.57 (1H, d, J = 15.3Hz, α-H ); 7.74 (1H, d, J = 15.3 Hz, β-H); 13.18 (1H, s, -OH). m + = 275.2.
Embodiment 2
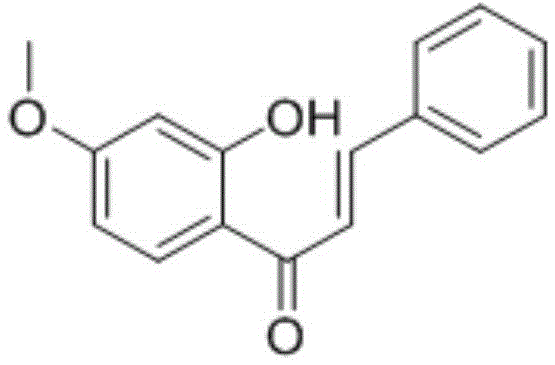
[0110] The preparation of embodiment 2 (E)-1-(2-hydroxyl-4-methoxyphenyl)-3-phenylprop-2-en-1-one (2)
[0111]
[0112] Using 2-hydroxy-4-methoxyacetophenone and benzaldehyde as raw materials, refer to the synthesis method of compound 1.
[0113] 1 H-NMR: 3.87(3H,s,-OCH 3 ),6.49(1H,s),6.52(1H,d,J=2.4,Ar-H),7.43(3H,m,Ar-H),7.67(1H,d,J=15.3Hz,Ar-H) ,7.65(2H,m,Ar-H),7.84(1H,d,J=9.3Hz),7.90(1H,d,J=15.3Hz),13.43(1H,s,-OH).M + = 255.2.
Embodiment 3
[0114] The preparation of embodiment 3 (E)-1-(2-hydroxyl-6-methoxyphenyl)-3-(thien-2-yl) prop-2-en-1-one (3)
[0115]
[0116] Using 2-hydroxy-6-methoxyacetophenone and thiophenecarbaldehyde as raw materials, refer to the synthesis method of compound 1.
[0117] 1 H-NMR: 3.07(3H,s,-OCH 3 ),6.43(1H,dd,J 1 =1.2,J 2 =8.4,Ar-H),6.61(1H,dd,J 1 =0.9,J 2 =8.1,Ar-H),7.10(1H,m,Ar-H),7.35(2H,m,Ar-H),7.41(1H,m,Ar-H),7.71(1H,d,J=15.3 ), 7.75 (1H, d, J = 15.3), 13.18 (1H, s, -OH). m + = 261.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com