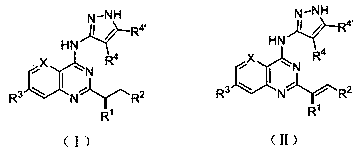
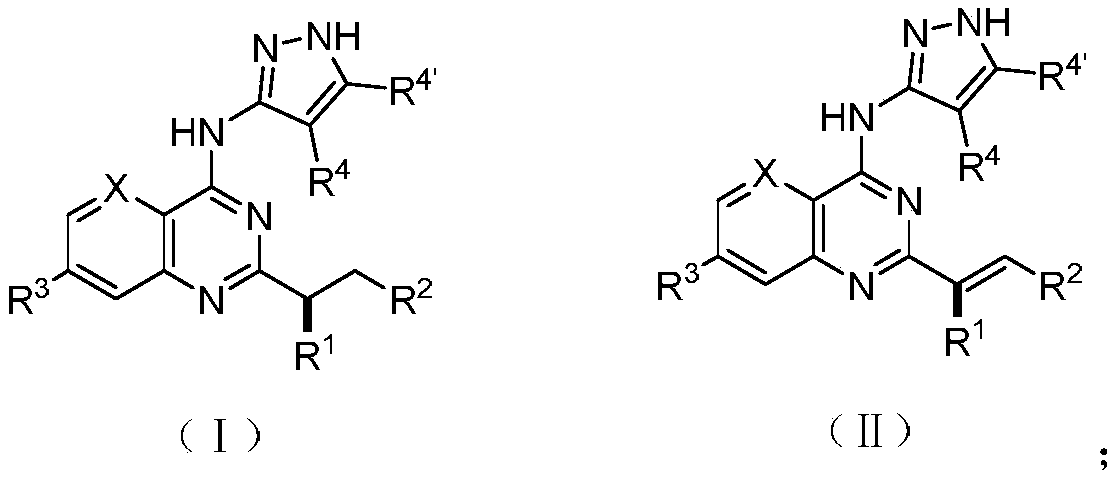
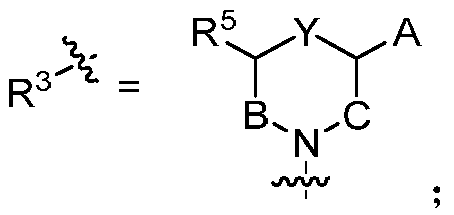
Substitute quinazolines derivative with Aurora kinase inhibitory activity and application thereof
A compound and hydrate technology, applied in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as arrhythmia and time difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] N-(5-methyl-1H-pyrazol-3-yl)-7-(4-methylpiperazin-1-yl)-4-amino-2-phenethylquinazoline
[0137]
[0138] Step 1: 4-Chloro-2-(3-phenylpropanamido)benzoic acid
[0139]
[0140] 2-Amino-4-chlorobenzoic acid (10.3g, 60mmol), potassium carbonate (24.9g, 180mmol) was added to 200mL of acetonitrile, and the freshly prepared phenylpropionyl chloride (72mmol) was slowly added dropwise, and the reaction was completed after the dropwise addition The temperature of the system was raised to 80°C, heated to reflux for 5 hours, a large amount of precipitates were formed in the reaction system, 1N hydrochloric acid solution was added to adjust the pH value to 2, about 100mL of dichloromethane was added, after vigorous stirring, the amide intermediate (12.4g, 68 %). 1 HNMR (400MHz, DMSO-d 6 )δ:13.89(s,1H),11.24(s,1H),8.59(s,1H),7.96(d,J=8.5Hz,1H),7.31-7.13(m,6H),2.93(t,J =7.4Hz, 2H), 2.73(t, J=7.4Hz, 2H). 13 CNMR (100MHz, DMSO-d 6 )δ: 170.9, 168.7, 141.8, 140.5, 138.4, 132.8...
Embodiment 2
[0151] 2-(1-amino-2-phenethyl)-N-(5-methyl-1H-pyrazol-3-yl)-7-(4-methylpiperazin-1-yl)quinazoline- 4-amine
[0152]
[0153] Step 1: 2-Amino-4-(4-methylpiperazin-1-yl)benzamide
[0154]
[0155] Dissolve 4-chloro-2nitrobenzonitrile (5.5g, 30mmol) and methylpiperazine (7.4mL, 66mmol) in 50mL of dioxane, reflux at 110°C for 16 hours, and remove the dioxane under reduced pressure. Add dichloromethane after the hexacyclic ring to extract, the organic phase is dried with anhydrous sodium sulfate, the intermediate obtained by concentration is dissolved in 100mL ethanol, wet Pd / C (300mg) and hydrazine hydrate (5.8mL, 120mmol) are added, and the reaction system is React overnight at 80 degrees, remove the organic solvent under reduced pressure, add MeOH:CH 2 Cl 2 (1:10) about 150mL solvent dissolved product, through diatomaceous earth column, MeOH:CH 2 Cl 2 (1:10) solvent washing, the solid obtained after the organic phase was concentrated was washed with a small amount of ...
Embodiment 3
[0163] N-(5-Methyl-1H-pyrazol-3-yl)-2-phenethylquinazolin-4-amine
[0164]
[0165] Step 1: 2-Phenylethylquinazolin-4(3H)-one
[0166]
[0167] Suspend 2-aminobenzamide (8.2g, 60mmol) and potassium carbonate (2equiv.) in dry acetonitrile, and gradually add phenylpropionyl chloride (9.8mL, 66mmol). Reflux for 5 hours, add 100mL of water, and obtain the intermediate by suction filtration. Dissolve the intermediate in 100mL of ethanol, add 10M NaOH (4 equiv.) solution dropwise at 0°C, stir at room temperature for 30 minutes, then use concentrated hydrochloric acid at 0°C After neutralization, a large amount of solids were precipitated. After removing an appropriate amount of ethanol under reduced pressure, 100 mL of water was added, and the target product (13.2 g, 88%) was obtained by suction filtration.
[0168] Step 2: N-(5-Methyl-1H-pyrazol-3-yl)-2-phenethylquinazolin-4-amine
[0169]
[0170] Dissolve 2-phenethylquinazolin-4(3H)-one (125 mg, 0.5 mmol), PyBrop (303 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com