A kind of purification method of sevoflurane
A purification method, the technology of sevoflurane, which is applied in the field of separation and purification of chemicals, can solve the problems of increasing production cost and complexity, and the separation effect is not very good, and achieves the effect of simple method and easy realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
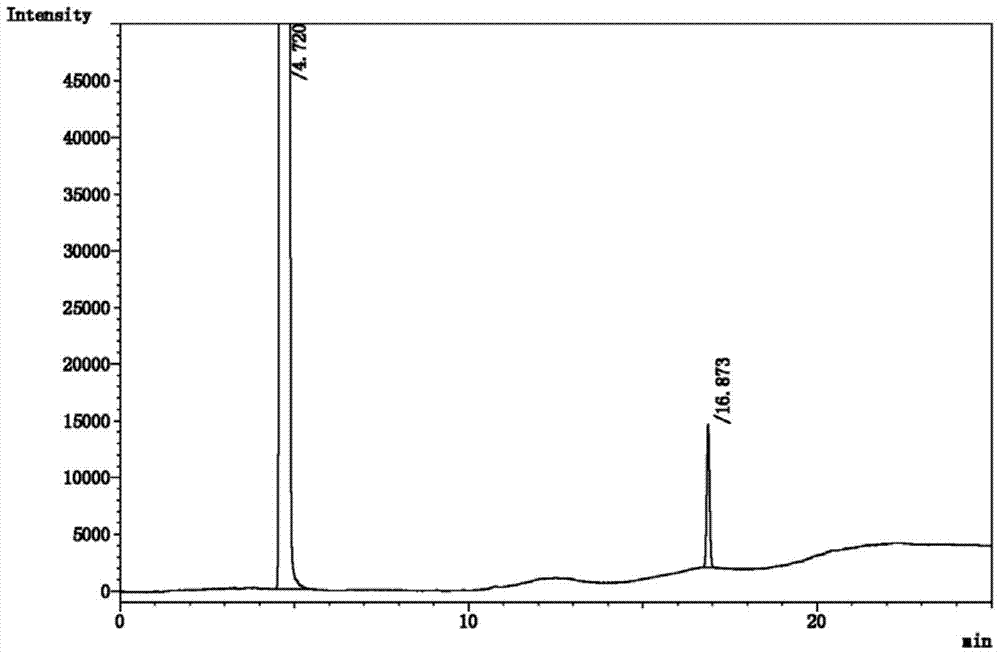
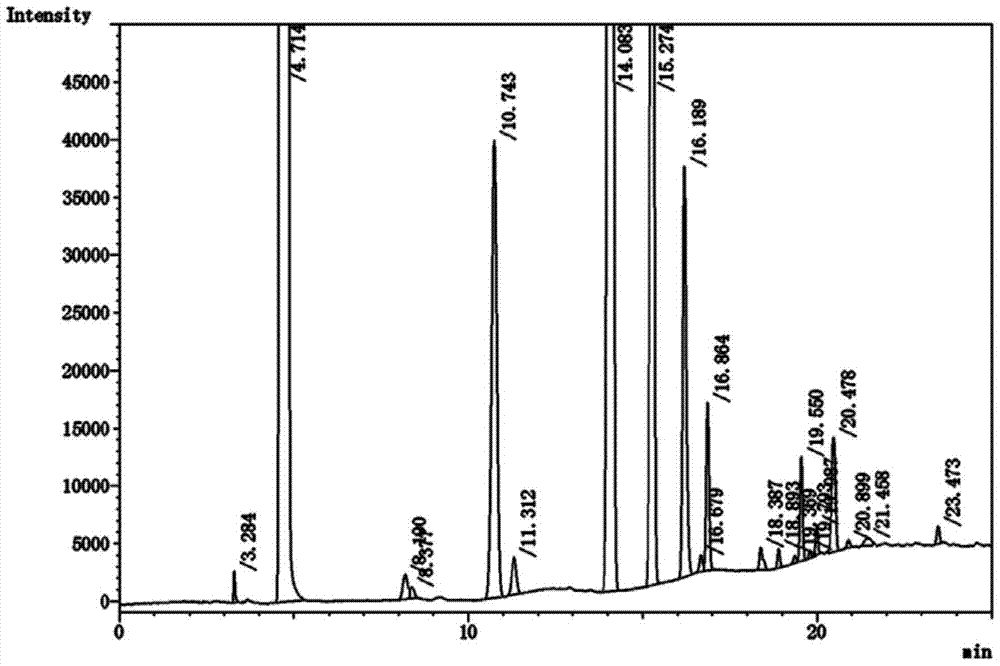
[0023] Using hexafluoroisopropanol and paraformaldehyde as raw materials, the crude product of sevoflurane was prepared by fluorination with potassium fluoride after the reaction under the catalysis of aluminum trichloride. After testing, the crude product of sevoflurane contained hexafluoroisopropanol , and other by-product impurities such as methyl hexafluoroisopropyl ether, chloromethyl hexafluoroisopropyl ether, chloromethyl-1,1,3,3,3-pentafluoro-2-propenyl ether, Polyether formed from fluoromethyl-1,1,3,3,3-pentafluoro-2-propenyl ether, hexafluoroisopropanol and paraformaldehyde [(CF 3 ) 2 CH(CH 2 O) n OCH(CF 3 ) 2 ] and some other unknown impurities.
[0024] The prepared crude sevoflurane is purified. The purification method includes: adding the crude sevoflurane to be purified into the rectification tank of the rectification tower for rectification and purification. The temperature of the rectification tank is 63 ° C, so that the crude sevoflurane Boil and reflux...
Embodiment 2
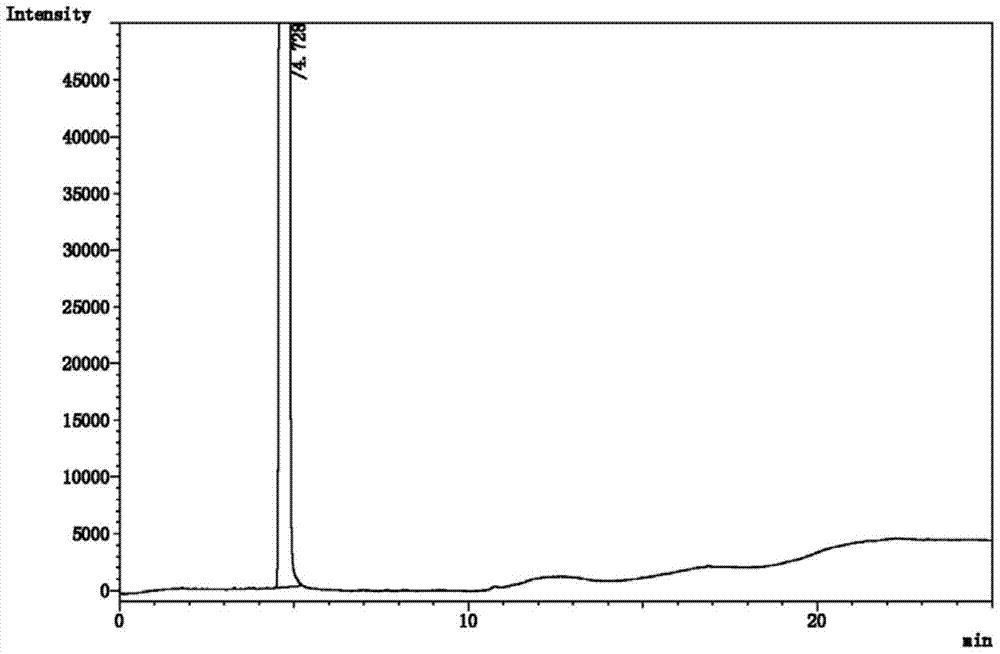
[0027] Purify the crude sevoflurane in Example 1. The purification method includes: adding the crude sevoflurane to be purified into the rectification tank of the rectification tower for rectification and purification. The temperature of the rectification tank is 65 ° C, making seven The crude halothane boils and refluxes in the rectification column. After the reflux reaches a stable level, control the reflux ratio to 5:1. Collect the previous fraction from the top of the rectification column. When the temperature at the top of the rectification column reaches 57°C, reduce the reflux ratio to 3. : 1, collect the positive fraction from the top of the rectification tower to obtain purified sevoflurane, and stop collecting when the temperature at the top of the rectification tower drops to 50°C; the rectification column is filled with stainless steel θ ring fillers with embedded silica gel , the stainless steel θ ring packing with embedded silica gel includes a stainless steel θ r...
Embodiment 3
[0031]Purify the crude sevoflurane in Example 1. The purification method includes: adding the crude sevoflurane to be purified into the rectification tank of the rectification tower for rectification and purification. The temperature of the rectification tank is 60 ° C, making seven The halothane crude product boils and refluxes in the rectification column. After the reflux reaches a steady state, control the reflux ratio to 3:1. Collect the front fraction from the top of the rectification column. When the temperature at the top of the rectification column reaches 53°C, reduce the reflux ratio to 2. : 1, collect the positive fraction from the top of the rectification tower to obtain purified sevoflurane, and stop collecting when the temperature at the top of the rectification tower drops to 50°C; the rectification column is filled with stainless steel θ ring fillers with embedded silica gel , the stainless steel θ ring packing with embedded silica gel includes a stainless steel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com