Preparation method of high-purity 3,6-dibromo-carbazole
A dibromocarbazole, high-purity technology is applied in the field of preparation of high-purity 3,6-dibromocarbazole, can solve the problems of many by-products, high toxicity, long reaction time, etc., achieves easy operation and control, is beneficial to Industrial production, the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
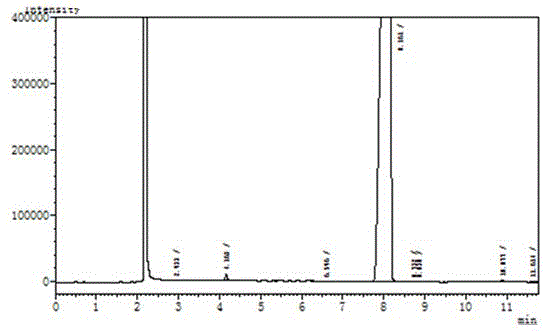
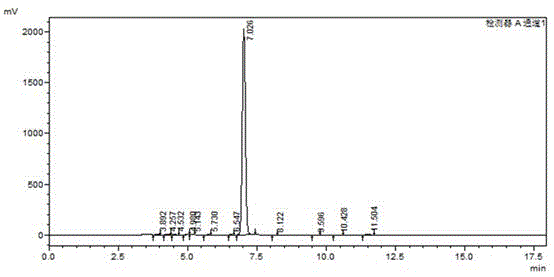
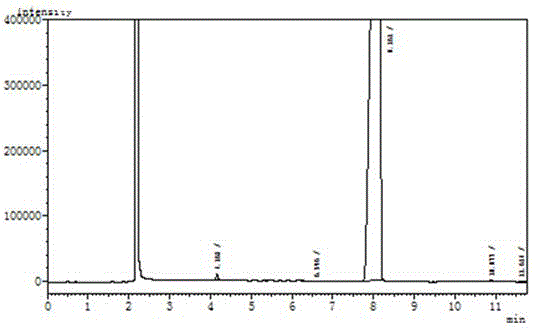
[0018] The detailed steps of the present invention are as follows: wherein, the feeding amount is as follows: carbazole: 167.2g, dibromohydantoin: 285.8g, absolute ethanol: 1800ml. Add 167.2g of carbazole (1mol) and 1000ml of absolute ethanol into a 2000ml reaction bottle, stir for 20 minutes to disperse evenly. Then add 285.8 g of dibromohydantoin in batches, control the reaction temperature at 20-25° C., and complete the addition in about 2 hours. After the addition, a large amount of white solids will precipitate out. Reaction was continued at this temperature for 1 hour. Use HPLC to monitor the reaction. When the monobrominated product in the reaction solution is less than 1%, stop the reaction and filter at 20-25°C. Add the filter cake to a 1000ml three-necked flask, then add 800ml of absolute ethanol, heat to reflux (80°C) for 1 hour, then slowly cool down to 20-25°C, filter, and dry under normal pressure at 100°C to obtain 310g of white powdery solid . Such as figur...
Embodiment 2
[0024] The detailed steps of the present invention are as follows: wherein, the feeding amount is as follows: carbazole: 167.2g, dibromohydantoin: 300.1g, absolute ethanol: 1800ml.
[0025] Add 167.2g of carbazole (1mol) and 1000ml of absolute ethanol into a 2000ml reaction bottle, stir for 20 minutes to disperse evenly. Then add 285.8 g (1.05 mol) of dibromohydantoin in batches, control the reaction temperature at 20-25°C, and complete the addition in about 2 hours. After the addition, a large amount of white solid will precipitate. Reaction was continued at this temperature for 1 hour. Use HPLC to monitor the reaction. When the monobrominated product in the reaction solution is less than 1%, stop the reaction and filter at 20-25°C. Add the filter cake to a 1000ml three-necked flask, then add 800ml of absolute ethanol, heat to reflux (80°C) for 1 hour, then slowly cool down to 20-25°C, filter, and dry under normal pressure at 100°C to obtain 312g of white powdery solid . S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com