A kind of pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., can solve problems such as poor fluidity, lack of API, and reduced standard tableting speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
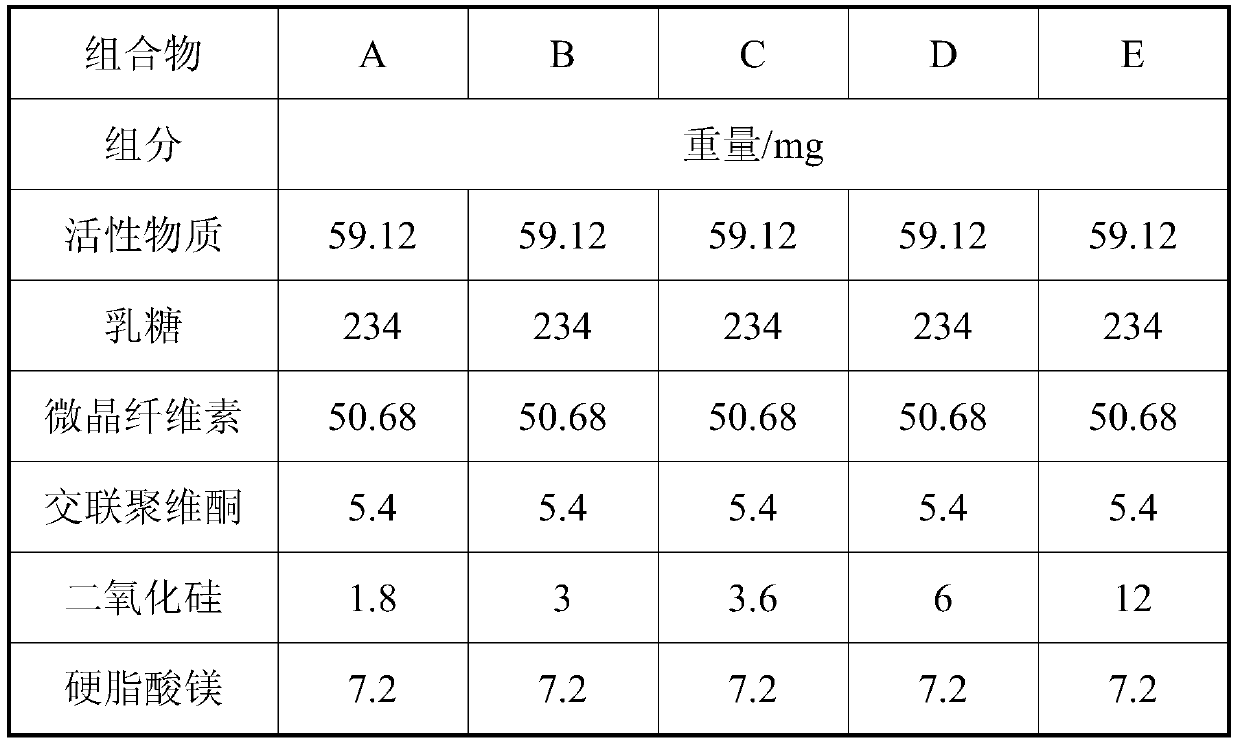
[0035] prescription:
[0036]
[0037] Taking materials according to the above prescription, mixing the active substance, lactose, microcrystalline cellulose and crospovidone to obtain an intermediate mixture, adding silicon dioxide and magnesium stearate and mixing to obtain a final mixture.
[0038] Directly observe the electrostatic state of each mixture powder in the whole process with the naked eye, and find that the intermediate mixtures are all electrostatically charged, but the electrostatic state of the final mixture of each prescription is different, and the measured particle content is different. The results are shown in Table 1 below.
[0039] Table 1 Experimental related results of Example 1
[0040] combination A B C D E character static electricity good good good static electricity particle content 93.2% 98.7% 99.8% 98.1% 94.9%
[0041] Note: The particle content in this article refers to the measured value / theoret...
Embodiment 2
[0044] Taking the composition C of Example 1 as an object, explore the impact of different mixing processes on the composition and the final tablet (directly made from composition C tablet compression), the technological scheme is summarized as follows:
[0045]
[0046] Note: Premix I, premix II, and final mixing are steps carried out in sequence, that is, add all the materials to the mixture obtained in the previous step, and then mix.
[0047] Result: It was observed that the compositions obtained after the total mixing of schemes 1 and 2 had no static electricity, but the intermediate mixture obtained from the premixing of scheme 1 had static electricity, while the intermediate mixture obtained from scheme 2 premix I and premix II had no static electricity. Static electricity; take 6 samples of the mixture obtained in each step, measure the particle content and calculate the deviation RSD, the results are shown in Table 2.1 and 2.2.
[0048] Table 2.1 Mixing Uniformity ...
Embodiment 3
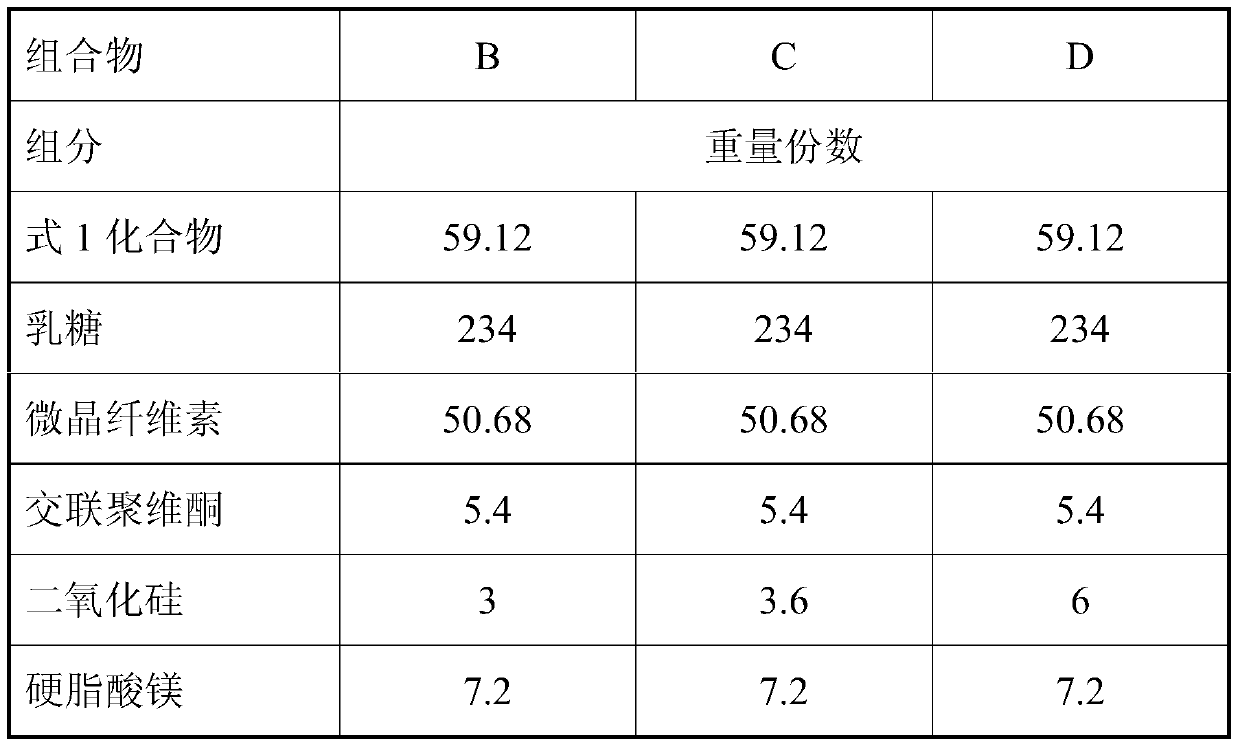
[0067] 1. Prescription
[0068] tablet Reference preparation C Element mg / tablet mg / tablet active substance 59.12 59.12 lactose 247.72 234 microcrystalline cellulose 36.96 50.68 Crospovidone 7.2 5.4 silica 1.8 3.6 Magnesium stearate 7.2 7.2
[0069] 2. Preparation process
[0070] 2.1 The samples of the reference preparation were prepared by dry granulation process;
[0071] 2.2 Tablet C uses the preparation process of Scheme 2 in Example 2 to prepare samples, and the hardness is controlled at 5-7kg / cm 2 .
[0072] 3. Dissolution
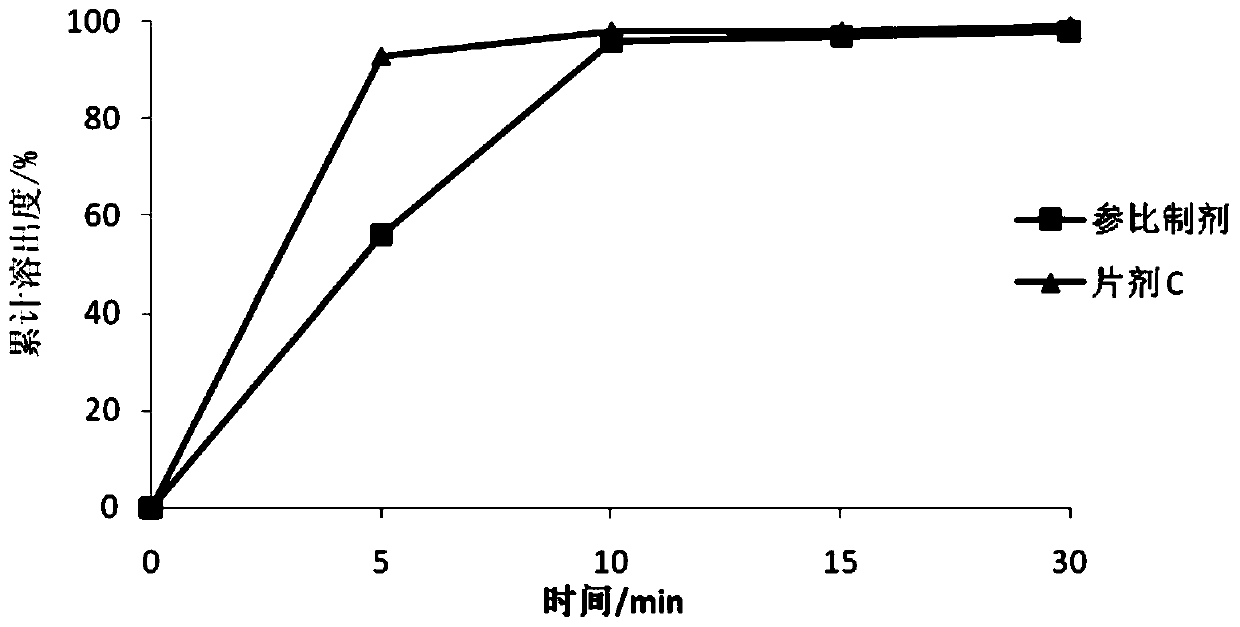
[0073] The dissolution rate of the tablet was determined according to the dissolution method used in the above table 2.3, and the results of the dissolution data are as follows:
[0074] time / min Reference preparation Tablet C 5 56% 93% 10 96% 98% 15 97% 98% 30 98% 99%
[0075] Dissolution curve comparison see figure 1 .
[0076] Clea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com