Universal artificial hapten and artificial complete antigen for capsaicins and application thereof
A technology of artificial haptens and complete antigens, which is applied in the field of immunochemistry to achieve the effects of strong specificity, high sensitivity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 capsaicinoid substance universal artificial hapten
[0029] Weigh 0.28 g of vanillin amine hydrochloride and dissolve it in 6 ml of tetrahydrofuran, add 0.15 g of triethylamine dropwise with stirring, and stir at room temperature for 30 min. Accurately weigh 0.15 g (0.0015 mol) of succinic anhydride and add to the above reaction solution, and stir overnight at room temperature. Add 3 mL of ethyl acetate to the reaction solution and stir at room temperature for 2 min. After filtration, the resulting precipitate is the hapten 4-[(4-hydroxy-3-methoxy)benzylamino]-4-carbonylcarboxylic acid (4-[ (4-hydroxy-3-methoxybenzyl)amino]-4-oxobutanoic acid), the molecular formula is C 12 h 15 NO 5 .
[0030] The results of NMR identification are: 1 HNMR(400MHz,DMSO)δ12.17(s,1H),8.87(s,1H),8.31(d,J=6.0Hz,1H),4.21(d,J=5.8Hz,2H),3.80(s, 3H), 2.52(t, J=6.9Hz, 2H), 2.42(t, J=6.7Hz, 2H). It is consistent with the theoretical value of the result, indic...
Embodiment 2
[0031] Example 2 Preparation of capsaicinoids universal artificial complete antigen-immune antigen
[0032] Weigh 20.3mg (about 0.08mmol) of the general artificial hapten of the above-mentioned capsaicinoids and 11.6mg (about 0.1mmol) of NHS in the reaction flask, add 400ulDMF to dissolve in the reaction flask, stir at room temperature for 30min, weigh 20.6mg (about 0.1mmol) 0.1 mmol) of DCC was dissolved in 100 ul of DMF, and the DCC / DMF solution was added dropwise to the above-mentioned reaction flask, stirred at room temperature for 4 h, and left standing overnight at 4°C. 8000rpm / 5min, take the supernatant active ester solution.
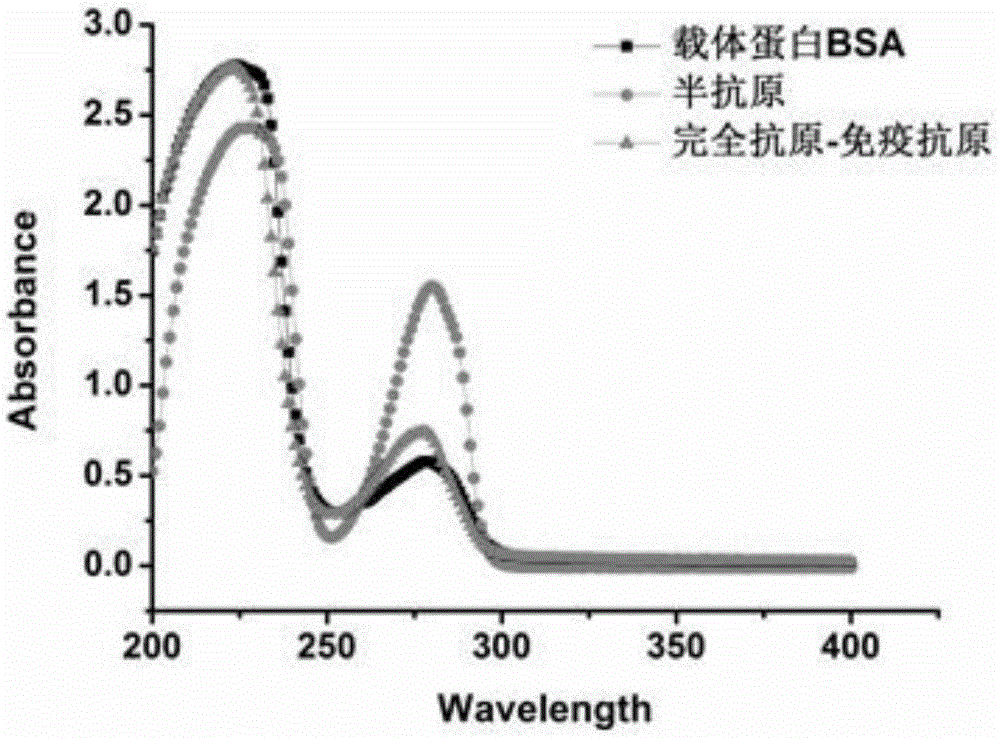
[0033] The supernatant active ester solution was added dropwise to 6ml of 7mg / ml BSA solution for reaction, and the reaction buffer was 0.2M phosphate buffer at pH 8.0. The reaction was carried out at room temperature for 4 h under magnetic stirring. The reaction solution was placed in a dialysis bag, and dialyzed with 0.01 M pH7.4 PBS at 4°C w...
Embodiment 3
[0034] Example 3 Capsaicinoids Universal Artificial Complete Antigen-Preparation of Coated Antigen
[0035] Dissolve 4.55 mg of the above-mentioned general artificial hapten of capsaicinoids in 200 μL of anhydrous DMF, then add 4.27 μL of tri-n-butylamine and 2.34 μL of isobutyl chloroformate in sequence, and stir at room temperature for 1 hour in the dark to obtain activated Capsaicinoid hapten solution.
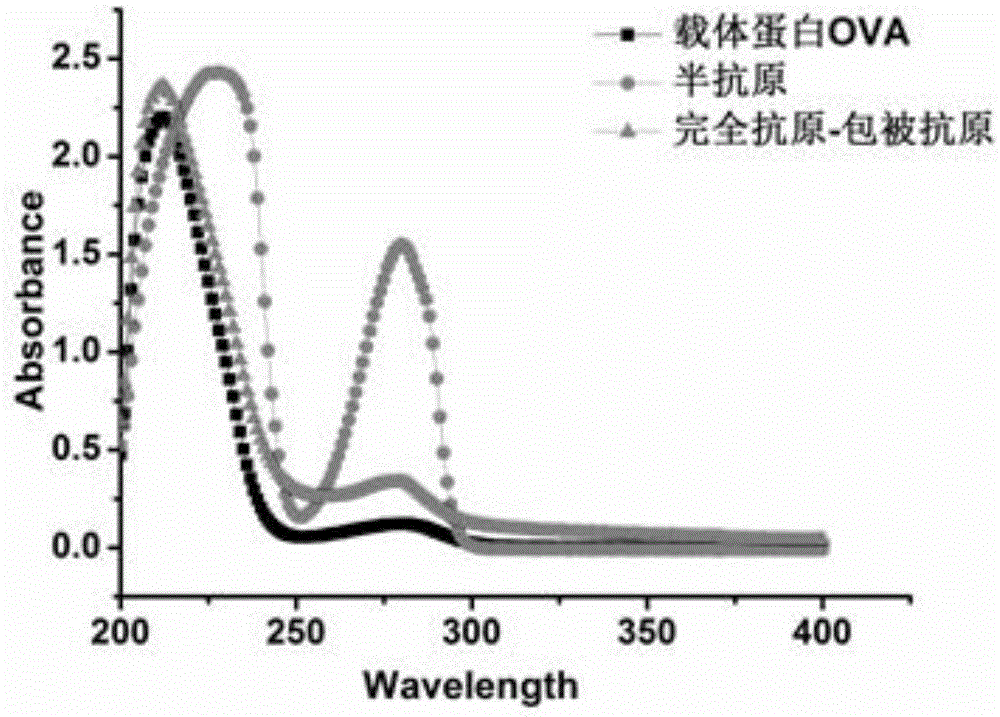
[0036] The activated capsaicinoid hapten solution was added dropwise to 10ml of 4.5mg / ml OVA solution for reaction, and the reaction buffer was 0.2M phosphate buffer at pH 8.0. The reaction was carried out at room temperature for 4 h under magnetic stirring. The reaction solution was placed in a dialysis bag, and dialyzed with 0.01M PBS, pH 7.4 at 4°C with stirring, and the dialysate was changed every 4 hours for a total of 72 hours. That is, capsaicin artificial antigen-coated antigen is obtained. For the continuous scanning spectrum of UV-Vis spectrum, see figure 2 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com