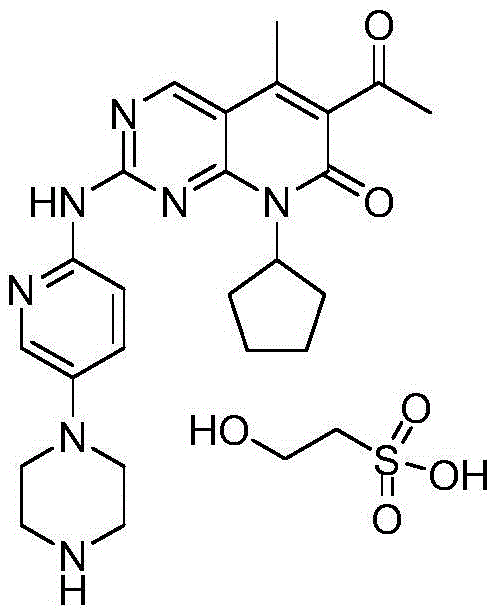
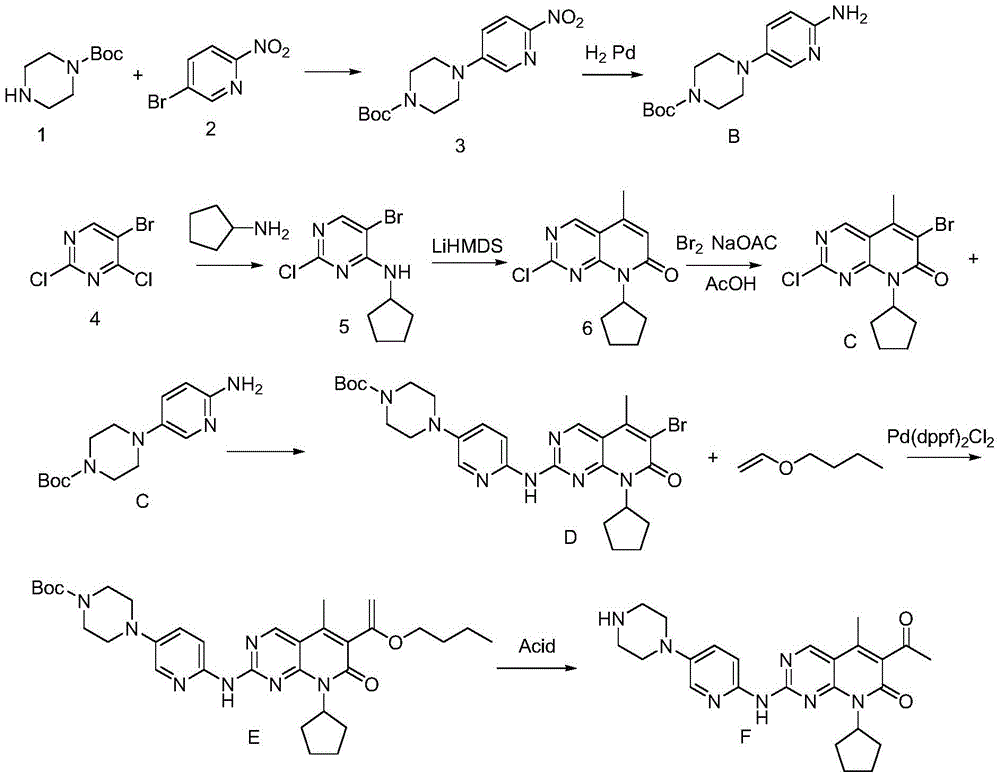
Preparation method of Palbociclib isethionate
A technology of isethionate and ethyl acetate, applied in the field of chemical drug synthesis, can solve the problems of unsuitability for industrial production, high production cost, long synthesis steps, etc., and achieves avoiding metal catalysts, reducing reaction costs and process conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preparation method of isethionate palbociclib (Palbociclib), comprising the following steps:
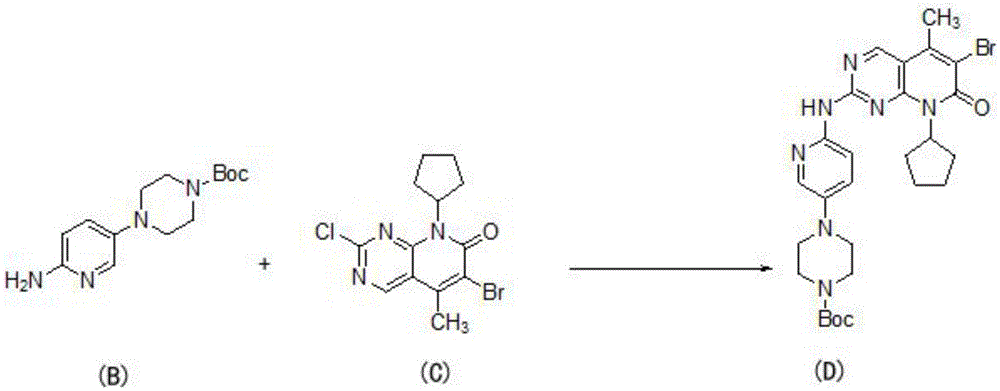
[0041] (1) Substituting the compound shown in formula B with the compound shown in formula C to obtain the compound shown in formula D:
[0042]
[0043]Put 810g (2.91mol) of compound B and 3.0LTHF into a 10L three-necked flask, evacuate and replace with nitrogen three times, stir at 20°C for 30 minutes, then slowly add 1.2L isopropylmagnesium chloride (2.0MinTHF) dropwise to the system, and keep warm at 20°C Stir for 1 hour, put 770g (2.25mol) of compound C into the system, evacuate nitrogen for 3 times, slowly add 1.2L isopropylmagnesium chloride (2.0MinTHF) dropwise to the system, after the dropwise addition, raise the temperature to 60°C The reaction was stirred, and the progress of the reaction was monitored by TLC. After the reaction, add 1.3L acetic acid and 1.3LTHF (tetrahydrofuran) mixed solution to the reaction solution, solids are precipitated, filtered by suc...
Embodiment 2
[0060] (1) Substituting the compound shown in formula B with the compound shown in formula C to obtain the compound shown in formula D:
[0061]
[0062] Put 810g (2.91mol) of compound B and 3.9LTHF into a 10L three-necked bottle, vacuum nitrogen replacement 3 times, stir at 20°C for 30 minutes, then slowly add 1.2L isopropylmagnesium chloride (2.0MinTHF) dropwise to the system, and keep warm at 20°C Stir for 1 hour, put 770g (2.25mol) of compound C into the system, evacuate nitrogen for 3 times, slowly add 1.2L isopropylmagnesium chloride (2.0MinTHF) dropwise to the system, after the dropwise addition, raise the temperature to 70°C The reaction was stirred, and the progress of the reaction was monitored by TLC. After the reaction, add 1.3L acetic acid and 1.3LTHF (tetrahydrofuran) mixed solution to the reaction solution, solids are precipitated, filtered by suction, the filter cake is beaten once with acetone, water, and acetone respectively, and air-dried at 50 ° C. The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com