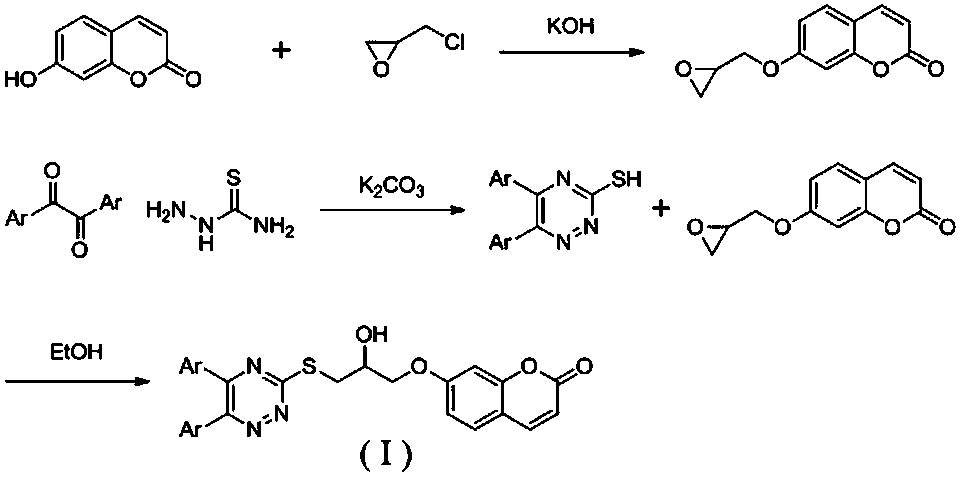
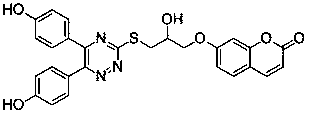
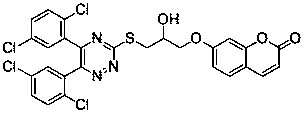
A kind of 1,2,4-triazine-coumarin type compound and its preparation method and application
A compound, coumarin technology, applied in organic chemistry, drug combination, metabolic diseases, etc., can solve the problems of high toxicity and side effects, hinder application, low activity, etc., and achieve a good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: 7-(3-((5,6-di(furan-2-yl)-1,2,4-triazin-3-yl)thio)-2-hydroxypropoxy)-2H - Preparation of benzopyran-2-one (1)
[0020]
[0021] Step 1: Put 7-hydroxycoumarin (0.58 g, 3.6 mmol), epichlorohydrin (4.75 g, 51.3 mmol), potassium hydroxide (0.25 g, 4.5 mmol) in a round bottom flask, add ethanol, The reaction was refluxed for 5 h, TLC showed that the reaction was complete, the reaction was stopped, cooled to room temperature, the filtrate was spin-dried, separated and purified by silica gel chromatography to obtain a white solid powder 7-(oxirane-2-ylmethoxy)-2H- Benzopyran-2-one, yield 96%.
[0022] Step 2: Mix 1,2-bis(furan-2yl)-ethane-1,2-dione (0.95 g, 5 mmol), thiosemicarbazide (0.46 g, 5 mmol), potassium carbonate (1.04 g , 7.5 mmol) in a round-bottomed flask, add 50 mL of water, reflux for 16 h, TLC shows that the reaction is complete, stop the reaction, cool to room temperature, adjust the pH to 5 with glacial acetic acid, and filter to obtain Brown-r...
Embodiment 2
[0025] Example 2: 7-(3-((5,6-diphenyl-1,2,4-triazin-3-yl)thio)-2-hydroxypropoxy)-2H-benzopyran - Preparation of 2-ketone (2)
[0026]
[0027] Yield 86%. 1 H NMR (d 6 -DMSO, 400 MHz) δ: 3.46 (dd, 1H), 3.65 (dd, 1H),4.15 (m, 3H), 5.69 (s, 1H), 6.28 (d, 1H), 6.92 (dd, 1H), 6.97 (d, 1H), 7.35(m, 10H), 7.60 (d, 1H), 7.97 (d, 1H); EIMS m / z = 484 [M + ].
Embodiment 3
[0028] Example 3: 7-(3-((5,6-bis(3-fluorophenyl)-1,2,4-triazin-3-yl)thio)-2-hydroxypropoxy)-2H - Preparation of benzopyran-2-one (3)
[0029]
[0030] Yield 80%. 1 H NMR (d 6 -DMSO, 400 MHz) δ: 3.45 (dd, 1H), 3.64 (dd, 1H),4.12 (m, 3H), 5.70 (s, 1H), 6.27 (d, 1H), 6.91 (dd, 1H), 6.96 (d, 1H), 7.23(m, 6H), 7.42 (m, 2H), 7.59 (d, 1H), 7.97 (d, 1H); EIMS m / z = 520 [M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com