Product for inhibiting and/or preventing influenza virus secondary bacterial infection
A technology for influenza virus infection and prevention of influenza, which is applied in the field of biomedicine and can solve problems affecting the prevention and control of severe influenza and death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
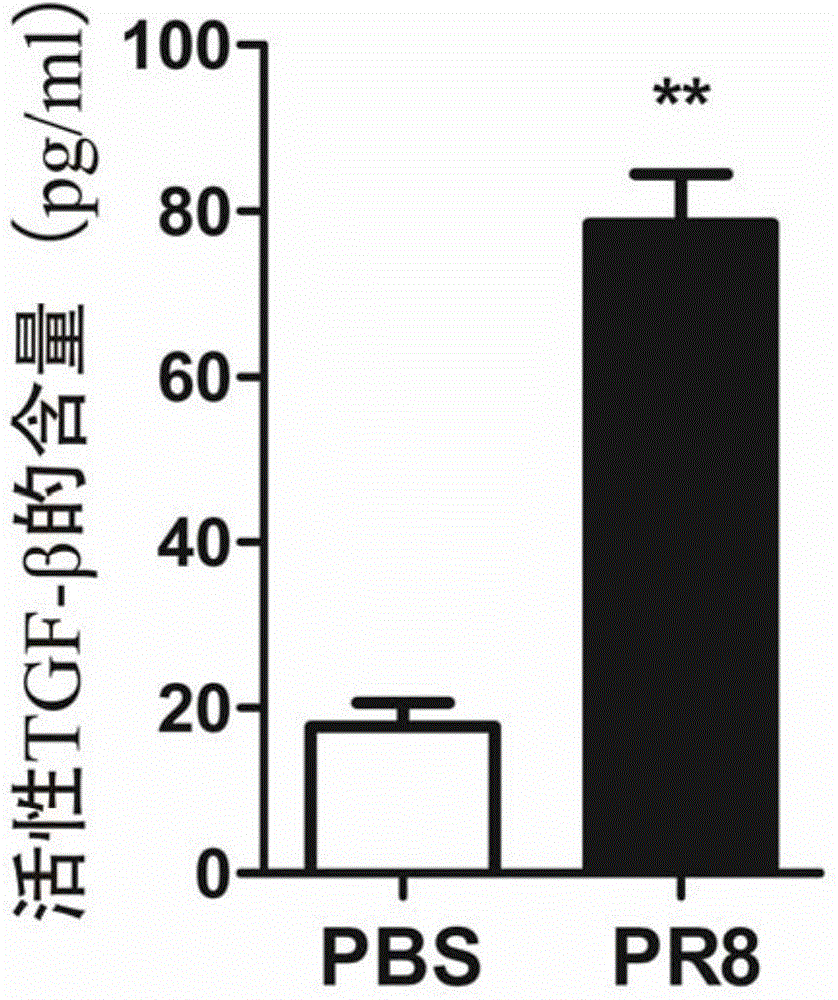
[0065] Example 1, IAV can promote the activity of human lung epithelial cells A549 secreting TGF-β
[0066] 1. Preparation of test samples
[0067] 1. Cultivate A549 cells, inoculate 48-well plate, 0.5-1×10 5 / 0.5ml per well. Place at 37°C, 5% CO 2 Cultivate overnight in an incubator;
[0068] 2. Cells grow to 90-100% confluence, washed 1-2 times with PBS;
[0069] 3. Add 270 μl / well serum-free DMEM medium;
[0070] 4. Add 30 μl / well H1N1 influenza virus PR8 virus solution (0.5-1×10 4 TCID50) or PBS control (30 μl / well PBS), after mixing. Place at 37°C, 5% CO 2 Cultivate in the incubator for 45min;
[0071] 5. Wash the cells 1-2 times with PBS to remove the virus liquid;
[0072] 6. Add 300 μl of DMEM complete culture solution, and collect the supernatant at different time points of culture, the time points are 2h, 4h, 6h;
[0073] 7. After the collected cell supernatant was centrifuged at 1000 g at 4°C for 10 minutes to remove cell debris, it was stored at -20°C or ...
Embodiment 2
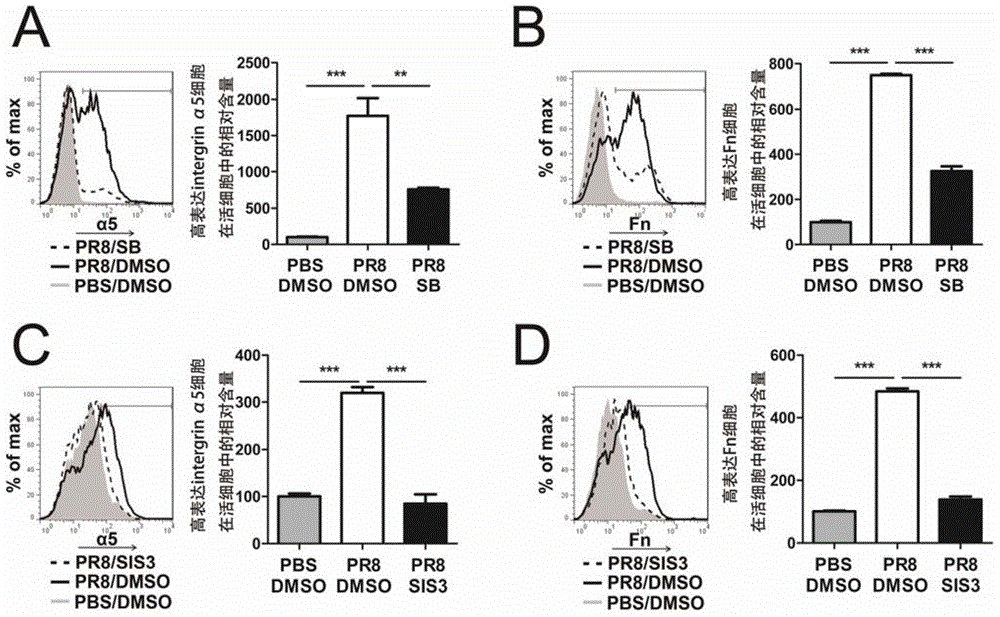
[0083] Example 2. IAV promotes the expression of integrin α5 and fibronectin on the surface of A549 cells, which can be inhibited by TGF-β signaling pathway inhibitor SB431542 or SIS3
[0084] 1. Preparation of test samples
[0085] 1. Culture A549 cells, press 1-2×10 5 / 1ml per well to inoculate a 24-well plate at 37°C 5% CO 2 Incubate overnight in an incubator;
[0086] 2. When the cells are 80-90% confluent, wash them twice with sterile PBS and add 0.5-1×10 4 The H1N1 influenza virus PR8 / 500μl of TCID50 per hole, the culture medium is serum-free DMEM (+4% (4g / 100mL) BSA); 50mM, dissolved in DMSO); the control group only added solvent (0.5μl DMSO). Place at 37°C 5% CO 2 Incubate for 18 hours in the incubator. At the same time, a control group treated as follows was set up in the experiment: no virus PR8 infection and only solvent (0.5 μl DMSO) was added, which was recorded as DMSO / PBS group.
[0087] 2. Flow Cytometry (FACS)
[0088] 1. 0.25% (0.25g / 100ml) trypsin 10...
Embodiment 3
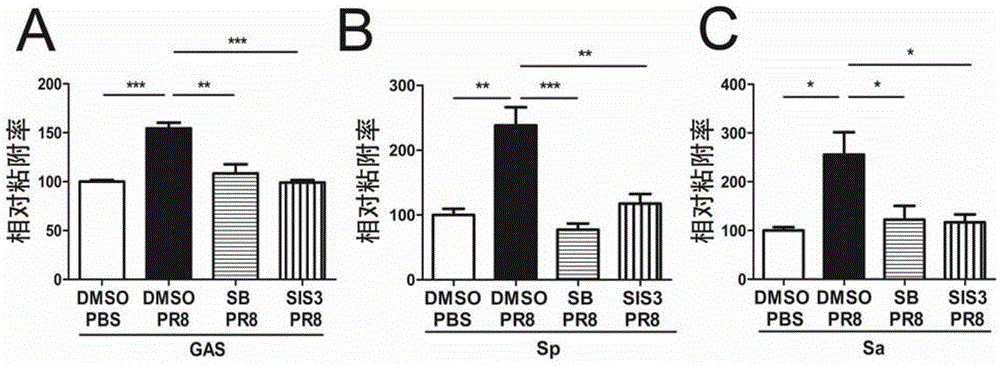
[0097] Example 3, IAV can promote the adhesion of type A streptococcus, pneumococcus and staphylococcus aureus to A549 cells, and this promoting effect can be inhibited by TGF-β signaling pathway inhibitor SB431542 or SIS3
[0098] 1. Infection of cells with PR8 virus
[0099] Same as Step 1 of Example 2.
[0100] 2. Bacterial adhesion test
[0101] Bacteria to be tested: Streptococcus type A (S.pyogenes) M1 type, Streptococcus pneumoniae (S.pneumococcus) T19F, Staphylococcus aureus (S.aureus) RN6390 (FnBPA+B+).
[0102] 1. Bacterial culture preparation: inoculate the bacteria into the corresponding culture medium (suspend with 10ml PBS for cultured colony, stand-by), place at 37°C 5% CO 2 Cultivate in the incubator for 17-18h. Centrifuge at 3000g for 10min, remove the culture medium (or PBS), and then add 10ml of PBS to wash once. Add an appropriate amount of serum-free DMEM (+4% (4g / 100mL) BSA), and adjust the amount of bacteria to 0.5-1×10 7 CFU / ml.
[0103] Among them,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com