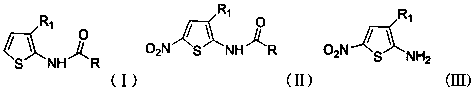
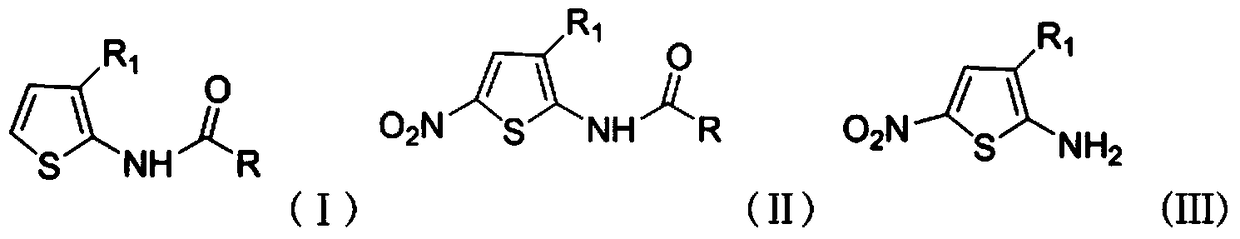
A kind of cu2+ catalyzes the method for preparing 2-amino-5-nitrothiophene compounds
A technology for the preparation of nitrothiophene and catalysis, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve the problem of high price of trifluoroacetic anhydride, limited large-scale production, Conditions are difficult to control and other problems, to achieve the effects of easy control of reaction temperature, simple and easy reaction operation, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] a Cu 2+ Process for the catalytic preparation of 2-amino-3-cyano-5-nitrothiophene.
[0030] The preparation method is as follows:
[0031] (1) Acetic anhydride-Cu 2+ / NO 3 - The preparation of compound salt system: add acetic anhydride 23ml (0.25mol) and Cu(NO 3 ) 2· 3H 2 O (12.08g, 0.05mol), the temperature control is 0°C, after stirring for 30min, the acetic anhydride-Cu 2+ / NO 3 - compound salt system;
[0032] (2) Nitration reaction: the acetic anhydride-Cu prepared towards step (1) 2+ / NO 3 - Slowly add 2-benzamide-3-cyanothiophene (11.4g, 0.05mol) into the compound salt system of , control the temperature range to 0-10°C, stir until the reaction is complete, pour the reaction solution into a large amount of ice water, Filtration, collected containing Cu 2+ The filtrate is set aside, and a dark solid is obtained, which is dissolved in ethanol and then decolorized with activated carbon. After the activated carbon is filtered off, recrystallization is c...
Embodiment 2
[0036] a Cu 2+ Process for the catalytic preparation of 2-amino-3-acetyl-5-nitrothiophene.
[0037] The preparation method is as follows:
[0038] (1) Acetic anhydride-Cu 2+ / NO 3 - The preparation of compound salt system: with embodiment 1.
[0039] (2) Nitration reaction: the acetic anhydride-Cu prepared towards step (1) 2+ / NO 3 - Slowly add 2-acetylamino-3-acetylthiophene (9.15g, 0.05mol) into the compound salt system of the compound salt system, control the temperature range to 10°C, stir until the reaction is completed, pour the reaction solution into a large amount of ice water, filter, and collect Contains Cu 2+ The filtrate was set aside, and a yellow solid was obtained, which was dissolved in methanol solution and then decolorized with activated carbon. After the activated carbon was filtered off, recrystallization was continued with the above methanol solution, and dark yellow crystals were precipitated. After filtration and drying, 2-aminoacetyl-3- Acetyl-...
Embodiment 3
[0042] a Cu 2+ Process for the catalytic preparation of 2-acetamido-3-acetamide-5-nitrothiophene.
[0043] The preparation method is as follows:
[0044] (1) Acetic anhydride-Cu 2+ / NO 3 - Preparation of the compound salt system: add acetic anhydride 23ml (0.25mol), CuSO 4 (7.98g, 0.05mol) and KNO 3 (5.06g, 0.05mol), the temperature control is 0 ℃, and after stirring for 30min, the acetic anhydride-Cu 2+ / NO 3 - compound salt system;
[0045] (2) Nitration reaction: the acetic anhydride-Cu prepared towards step (1) 2+ / NO 3 - Slowly add 2-acetamido-3-acetamide thiophene (9.20g, 0.05mol) to the compound salt system, control the temperature range to 5°C, stir until the reaction is complete, pour the reaction solution into a large amount of ice water, filter, and collect Contains Cu 2+ The filtrate was set aside, and a yellow solid was obtained, which was dissolved in methanol solution and then decolorized with activated carbon. After the activated carbon was filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com