Method for detecting pancreatic tumor, antibody, and pancreatic tumor detection kit
A detection method and antibody technology, applied in chemical instruments and methods, measuring devices, anti-animal/human immunoglobulin, etc., can solve problems such as high practical barriers, difficult signal attribution, and unsuitability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0068] The first embodiment of the present invention is an anti-APOA2 antibody (comprising an anti-APOA2 protein terminal antibody and an anti-APOA2 protein non-terminal antibody) and fragments thereof.
[0069] 1-1. Anti-APOA2 antibody
[0070] In the present specification, the term "APOA2 protein" refers to APOA2 proteins of various species, but preferably human-derived APOA2 proteins (GenBank Accession No. NP_001634.1). Specifically, it includes the human-derived wild-type APOA2 protein variant shown in SEQ ID NO: 1, 2 or 3, and further includes natural mutants and fragments thereof.
[0071] In this specification, the aforementioned "variants" refer to different molecular forms of the APOA2 protein that can exist in plasma, serum or other body fluids of humans or animals. For example, APOA2 proteins that differ in the structure of the C-terminal region in APOA2 proteins or natural mutants thereof. Specifically, APOA2 protein variants, for example, the APOA2-ATQ protein s...
Embodiment 1
[0209] (Example 1) Production of a monoclonal antibody (anti-APOA2-ATQ terminal monoclonal antibody) that specifically recognizes the C-terminal region of the APOA2-ATQ protein
[0210] (a) Production of cells that produce antibodies that recognize the C-terminal region of APOA2-ATQ protein
[0211] A peptide composed of the amino acid sequence shown in SEQ ID NO: 29, which is the sequence of the C-terminal region of the APOA2-ATQ protein, is poorly soluble in water and has low antigenicity, so it was synthesized by adding 3 arginines to the N-terminal side. residue to impart hydrophilicity, and a cysteine residue was added to its N-terminus. Next, maleimide-activated ovalbumin (Maleimide-Activated Ovalbumin, manufactured by Pias Co., Ltd.) was used to bind the cysteine residue of the aforementioned peptide to the OVA protein. Using this as an immunogen, 100 µg of the immunogen was intraperitoneally administered to mice (BALB / c) at intervals of 2 weeks. From the first im...
Embodiment 2
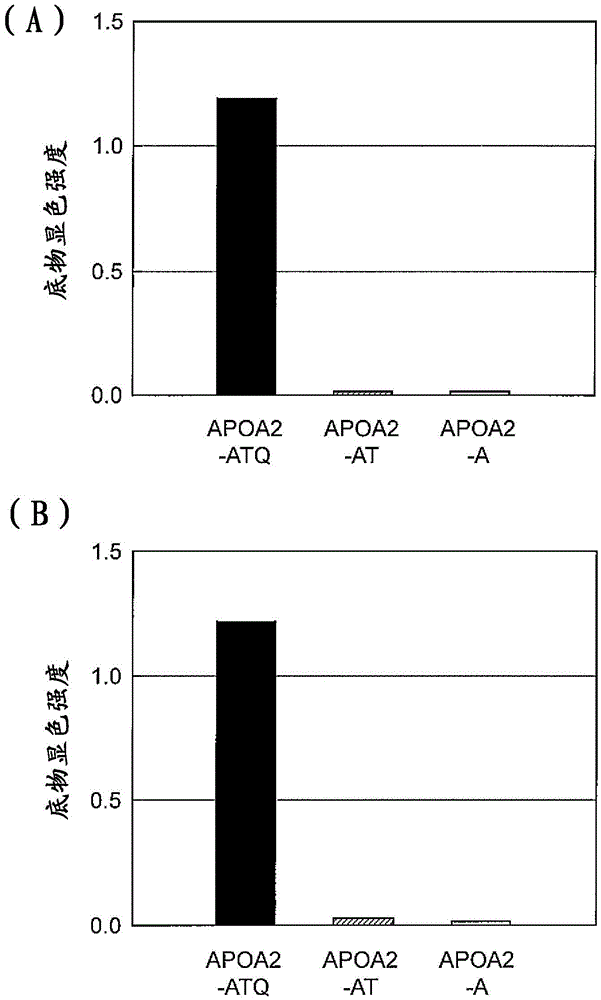
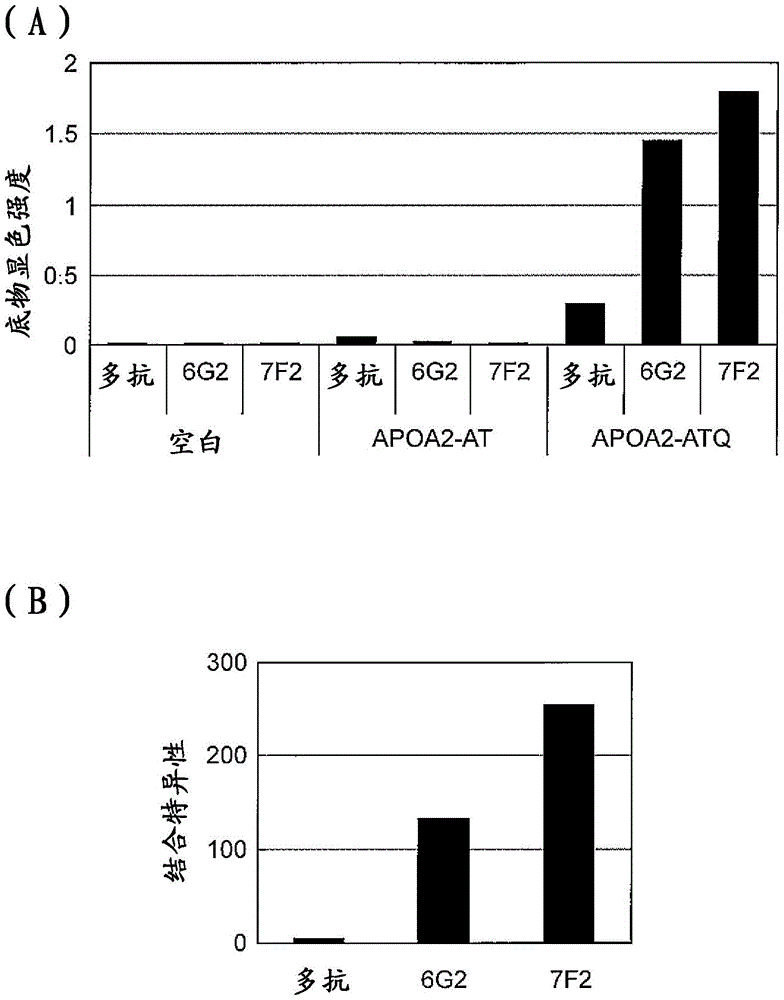
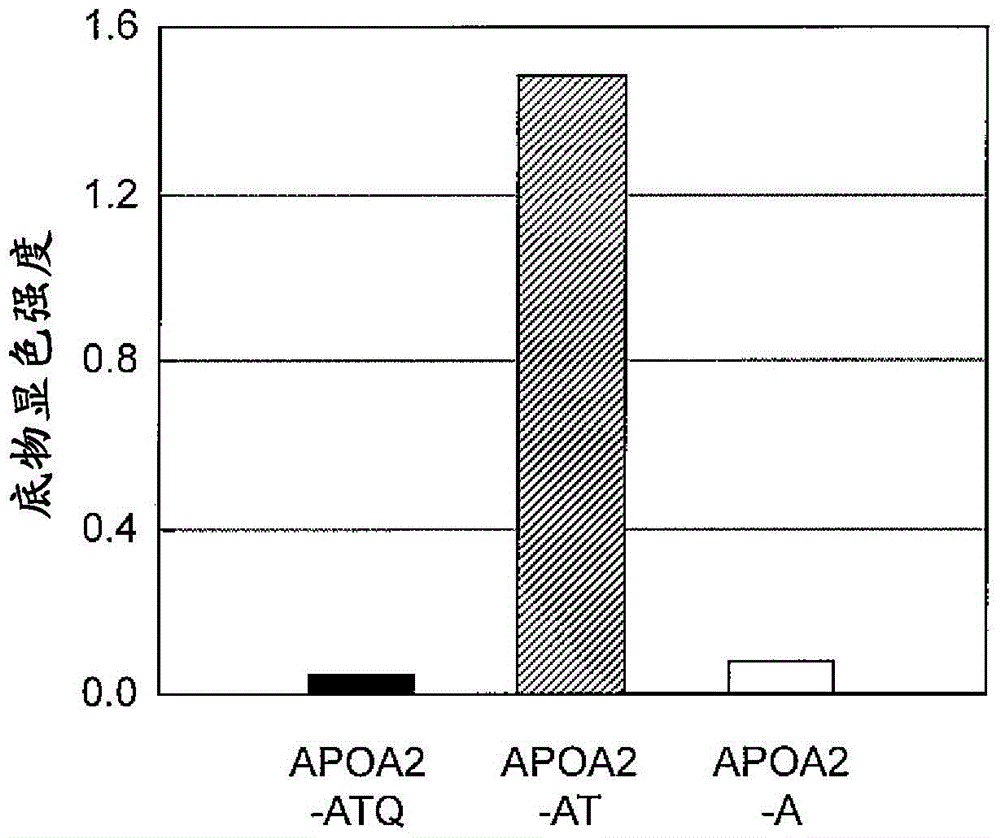
[0217] (Example 2) Detection of APOA2-ATQ protein by ELISA method using anti-APOA2-ATQ terminal monoclonal antibody
[0218] APOA2-ATQ protein was detected by ELISA using the anti-APOA2-ATQ terminal monoclonal antibody 7F2 or 6G2 obtained in Example 1. The recombinant human-derived APOA2-ATQ protein, APOA2-AT protein or APOA2-A protein was adjusted to 1 μg / mL with PBS solution, and then 100 μL was added to the wells of the immunoplate Maxisorp, and immobilized overnight. On the next day, the aforementioned solution was discarded, 400 μL of blocking buffer A solution was added, and the mixture was left to stand at room temperature for 1 hour. After discarding the solution in the well, add 400 μL of PBS-T for washing, and add 100 μL of antibody 7F2 or 6G2, react at room temperature for 2 hours. After discarding the solution in the well, it was washed with PBS-T, and then 100 μL of polyclonal rabbit anti-mouse immunoglobulin / HRP diluted 5000 times with a diluent was added and r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com