Aspergillomarasmin compound and synthesis method thereof
A technology of amine compound and synthesis method, which is applied in the direction of organic chemical method, chemical instrument and method, preparation of organic compound, etc., can solve the problems of difficult tramamine product, low output, limited source, etc., and achieves mild conditions and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] A kind of synthetic method of tramamide compound, synthetic route is as follows:
[0113]
[0114] , including the following steps:
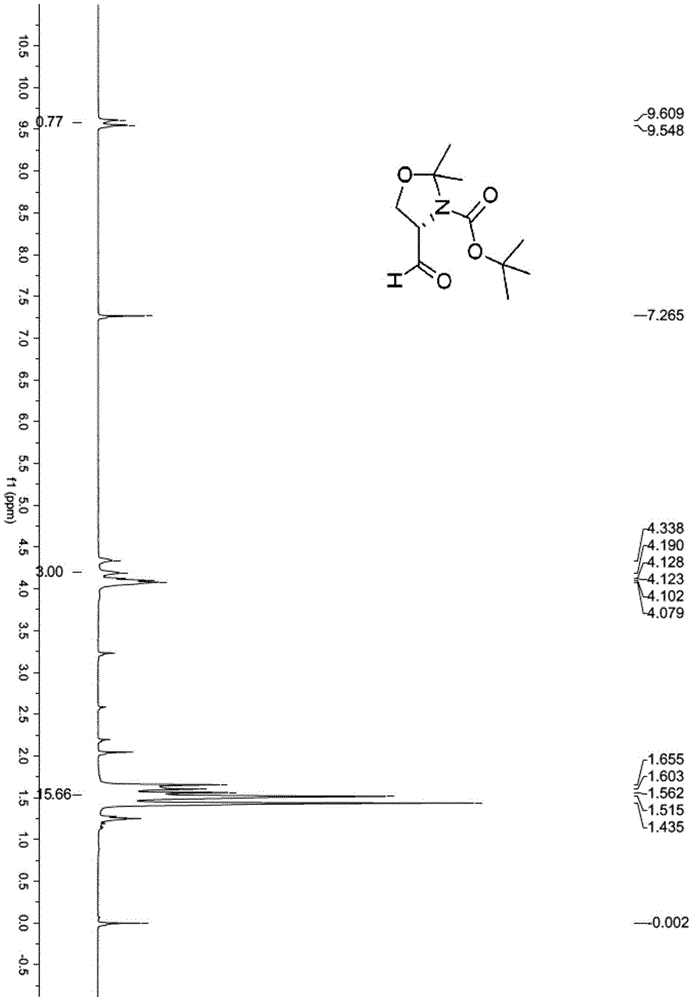
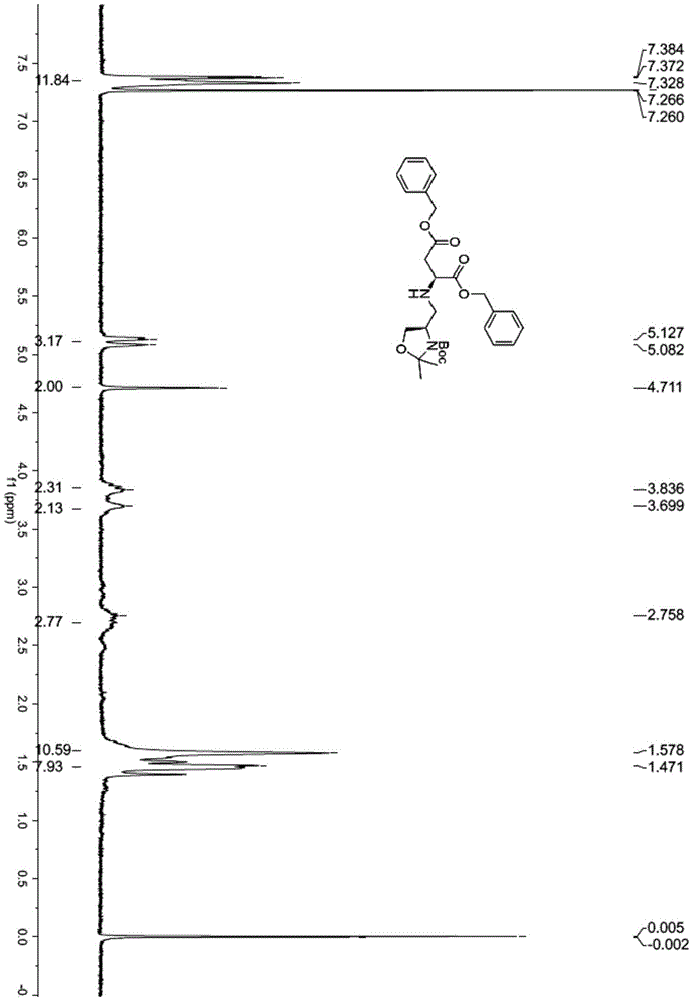
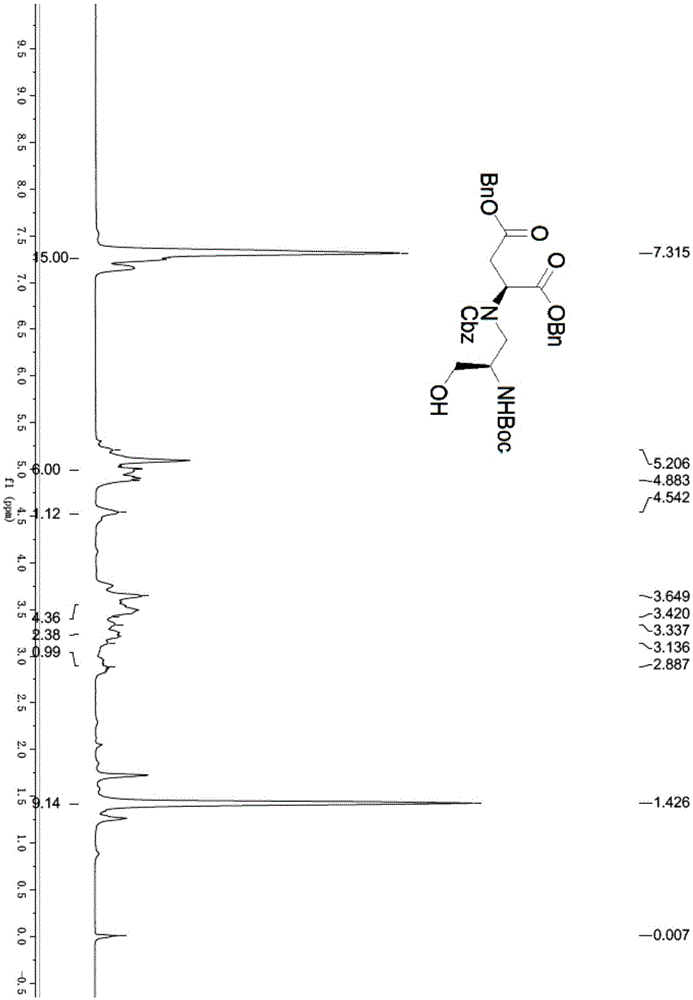
[0115] (1) Dissolve N-Boc-D-serine methyl ester (10g, 45.6mmol, purity 98%) in acetone (165mL), 2,2-dimethoxypropane (50mL, 400mmol) and boron trifluoride ether (0.35 mL, 2.8 mmol) were added sequentially. After stirring at room temperature for 2.5 h, TLC detected that the raw material had reacted. Triethylamine (0.9 mL) was added to quench the reaction, and then the solvent was evaporated under reduced pressure, ether (150 mL) and saturated sodium bicarbonate solution (250 mL) were added to separate the layers. The aqueous phase was extracted with diethyl ether (150 mL×2), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain compound 2 (12.8 g) as a pale yellow oil, which was directly used in the next step. 1HNMR (400MHz, CDCl 3 )δ4.51-4.35(m,1H),4.2...
Embodiment 2
[0130] Adopt the method identical with embodiment 1, synthesize a kind of chiral structure is the trameramine compound of SRS, synthetic route is as follows:
[0131]
Embodiment 3
[0133] Using the same method as in Example 1, a kind of chiral structure is synthesized as a trameramide compound of SSR, and the synthetic route is as follows:
[0134]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com