Preparation method of iron-chitosan metal supermolecular gel
A technology of supramolecular gel and chitosan, which is applied in the field of preparation of iron-chitosan gel and iron-chitosan metal supramolecular gel, can solve the problem that chitosan-iron supramolecular materials cannot Problems such as formation and disturbance of metal-supramolecular coordination, etc., to achieve excellent biological application prospects, simple preparation process, and extended application effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Preparation of iron-chitosan supramolecular gel:
[0039] (1) Preparation of chitosan solution:
[0040] Add 1g of acetic acid to 100mL with water, then dissolve 1g of chitosan in the above solution, stir well and let it stand overnight for use.
[0041] (2) Preparation of iron-chitosan supramolecular gel:
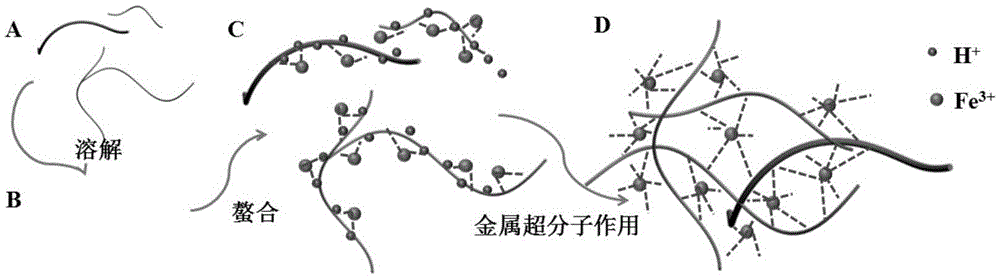
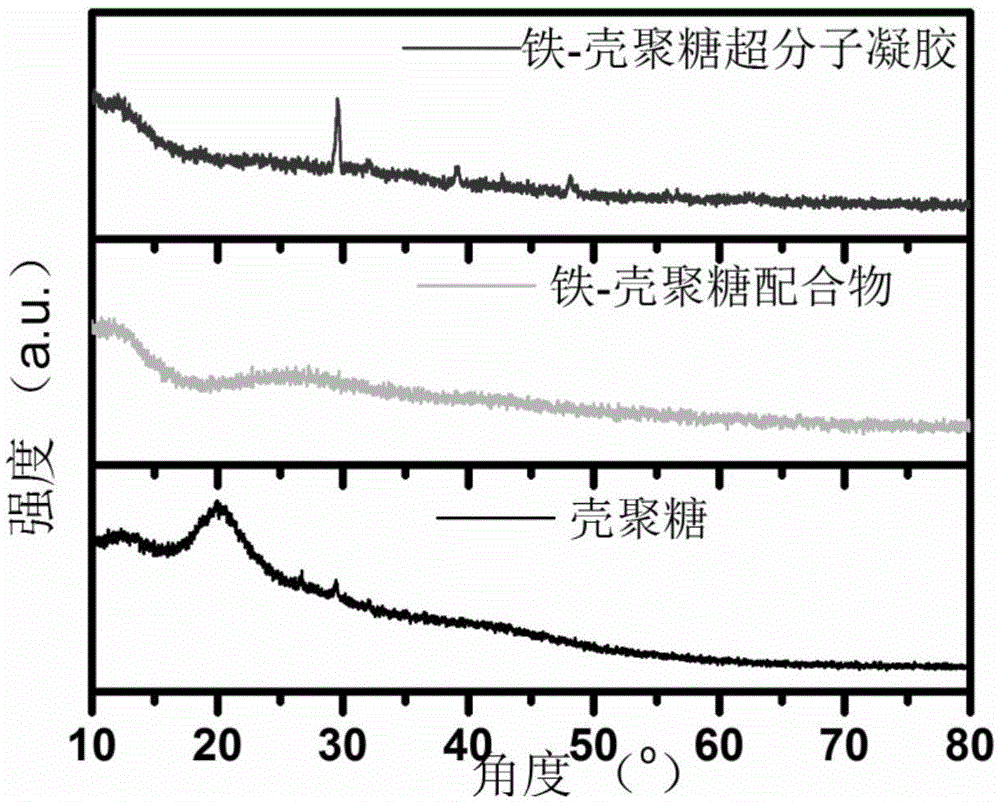
[0042] Mix 100 μL 0.5M ferric nitrate aqueous solution and 800 μL chitosan solution under vigorous shaking to ensure that the iron ions and chitosan molecules are fully coordinated to form a coordination compound, and then quickly add 100 μL 0.4M hydrogen under vigorous shaking Sodium oxide solution was used to deprotonate chitosan molecules and trigger metal-supramolecular interactions between iron ions and chitosan molecules to form iron-chitosan supramolecular gels. After standing at room temperature for 24 hours, the iron-chitosan supramolecular gel was successfully formed.
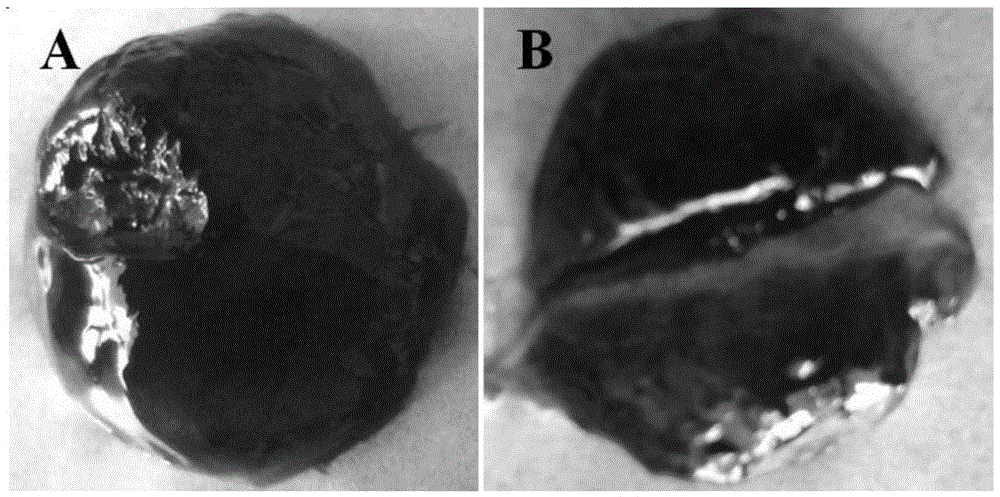
[0043] figure 1 It is a macroscopic view of the iron-chitosan supramolecular gel...
Embodiment 2
[0056] 1. Preparation of iron-chitosan supramolecular gel:
[0057] (1) Preparation of chitosan solution:
[0058] Add 1 g of acetic acid to 100 mL of water, then dissolve 2 g of chitosan in the above solution, stir well and let stand overnight for use.
[0059] (2) Preparation of iron-chitosan supramolecular gel:
[0060] Mix 100 μL 0.7M ferric nitrate aqueous solution and 800 μL chitosan solution under vigorous shaking to ensure that the iron ions and chitosan molecules are fully coordinated to form a coordination compound, and then quickly add 200 μL 0.6M hydrogen under vigorous shaking Sodium oxide solution was used to deprotonate chitosan molecules and trigger metal-supramolecular interactions between iron ions and chitosan molecules to form iron-chitosan supramolecular gels. After standing at room temperature for 24 hours, the iron-chitosan supramolecular gel was successfully formed.
[0061] 2. Self-healing and pH-responsive behavior research
[0062] (1) A block-sh...
Embodiment 3
[0067] 1. Preparation of iron-chitosan supramolecular gel:
[0068] (1) Preparation of chitosan solution:
[0069] Add 1g of acetic acid to 100mL with water, then dissolve 4g of chitosan in the above solution, stir well and let it stand overnight for use.
[0070] (2) Preparation of iron-chitosan supramolecular gel:
[0071] Mix 100 μL 0.9M ferric nitrate aqueous solution and 800 μL chitosan solution under vigorous shaking to ensure that the iron ions and chitosan molecules are fully coordinated to form a coordination compound, and then quickly add 300 μL 0.8M hydrogen under vigorous shaking Sodium oxide solution was used to deprotonate chitosan molecules and trigger metal-supramolecular interactions between iron ions and chitosan molecules to form iron-chitosan supramolecular gels. After standing at room temperature for 24 hours, the iron-chitosan supramolecular gel was successfully formed.
[0072] 2. Self-healing and pH-responsive behavior research
[0073] (1) A block-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com