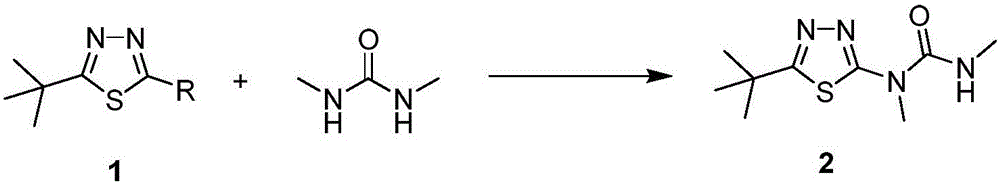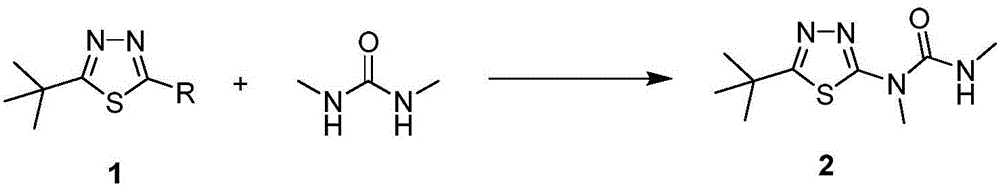Tebuthiuron synthesis method
A technology of tebuthiuron and synthetic method, which is applied in the field of organic synthesis, and can solve the problems of high toxicity of reaction raw materials, high safety risks, and difficulties in industrial operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 200g of toluene, 100g (0.54mol) of 5-tert-butyl-2-chloro-1,3,4-thiadiazole, 52.6g (0.59mol, 1.1eq) of 1,3-dimethylurea into the reaction flask , stir evenly, heat up to 60°C, add dropwise 30% aqueous sodium hydroxide solution 86g (0.65mol, 1.2eq), continue to insulate for 5 hours after the dropwise addition, complete the reaction, separate layers, continue to wash the toluene phase twice, and remove the solvent. About 150g of toluene was extracted, cooled to 0°C and left to stand for 6 hours, the product precipitated, and 120g of white solid was obtained by suction filtration, which was verified as Tebuthiauron by mass spectrometry and hydrogen spectrometry, and its purity was 97% by HPLC, and the yield was 94.4 %.
Embodiment 2
[0027] Add 300g of chlorobenzene, 100g (0.54mol) of 5-tert-butyl-2-chloro-1,3,4-thiadiazole, 55g (0.62mol, 1.15eq) of 1,3-dimethylurea into the reaction flask , Potassium Carbonate 90g (0.65mol, 1.2eq), stir evenly, slowly heat up to 50°C, keep warm for 3 hours, after the reaction is complete, add water and stir to separate layers, continue to wash the chlorobenzene phase twice with water, and precipitate about 250g of chlorobenzene , cooled to 10°C and left to stand for 4 hours, the product precipitated, and 115g of white solid was obtained by suction filtration, which was verified as Tebuthiauron by mass spectrometry and hydrogen spectrometry, and its purity was 96% by HPLC, and the yield was 90.0%.
Embodiment 3
[0029] Add 500 g of dichloromethane, 100 g (0.54 mol) of 5-tert-butyl-2-chloro-1,3,4-thiadiazole, 57.4 g (0.64 mol, 1.2 eq), stir evenly, slowly raise the temperature to 40°C, add 77g (0.77mol, 1.4eq) of triethylamine dropwise, and keep warm for 8 hours. , about 450g of dichloromethane was released, cooled to 20°C and left to stand for 4 hours, the product was precipitated, and 113g of white solid was obtained by suction filtration, which was verified as Tebuthiauron by mass spectrometry and hydrogen spectrometry, and its purity was 96% as detected by HPLC. Yield 88.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com