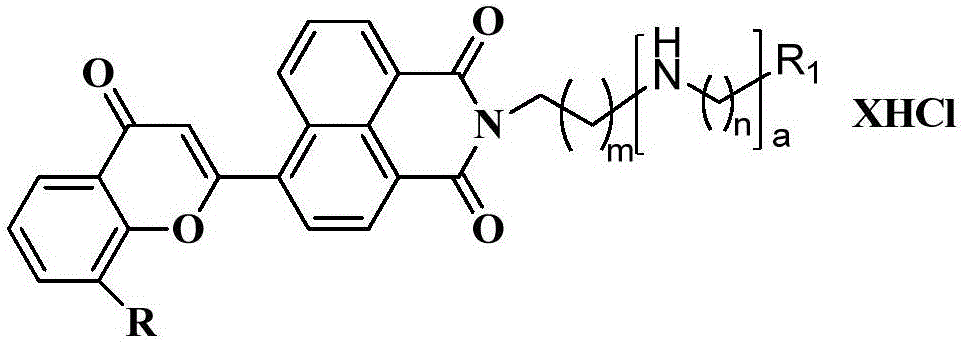
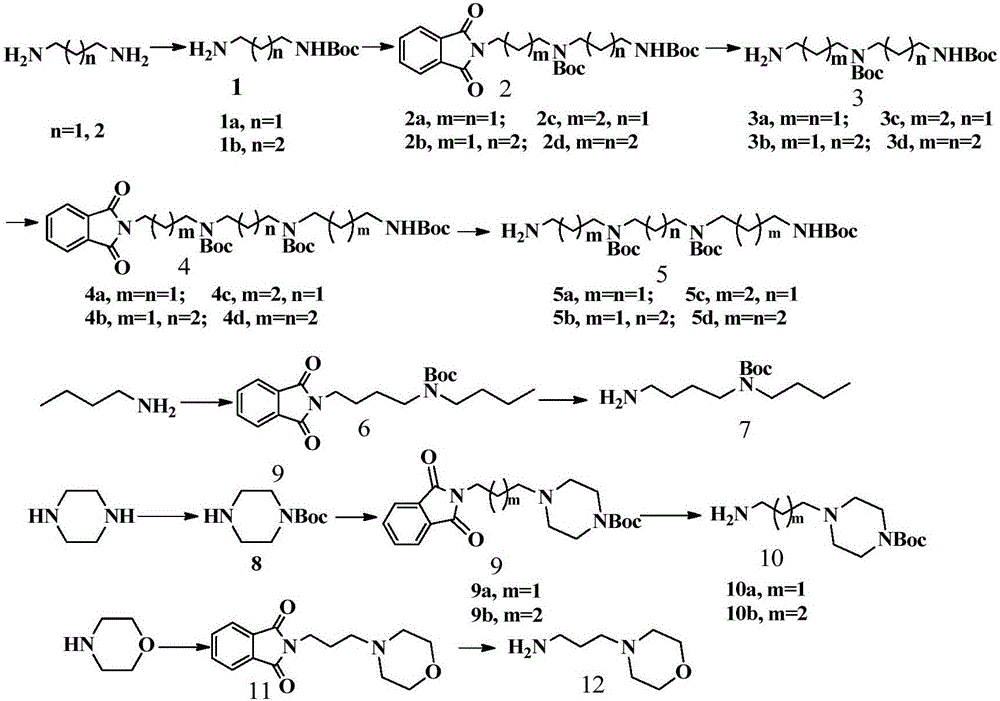
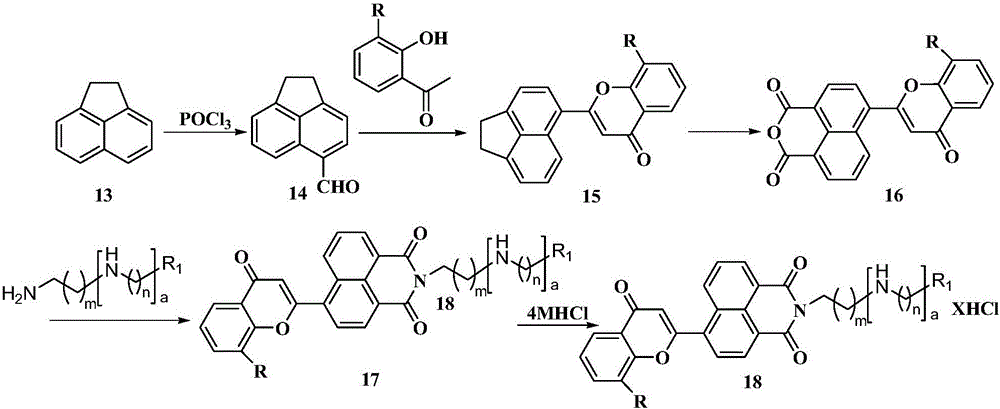
Benzopyran-4-one substituted naphthalimide-polyamine conjugate and preparing method and usage thereof
A technology of benzopyran and naphthalimide, applied in the field of "naphthalimide-polyamine" conjugates and its preparation, can solve problems such as limited clinical activity, toxic side effects, and central nervous system toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 6-(2-4H-1-benzopyran-4-one)-{2-[(2-dimethylamino)-ethyl]}-1H-benzoisoquinoline-1,3(2H )-diketone} hydrochloride (18a) preparation:
[0034]
[0035] (1) Dissolve 1,3-propanediamine (50mmol) in 30mL of 10v% methanol solution of triethylamine, and dissolve 4.3g of Boc 2 O (20mmol) methanol solution 20mL was slowly dropped into the above solution, and the drop was completed and raised to room temperature and stirred for 12h; 2 CO 3 Washed with solution, collected organic layer, anhydrous Na 2 SO 4 Dried and concentrated to give compound 1;
[0036] (2) Dissolve 4.50g (23.9mmol) of compound 1 in 100mL of acetonitrile, add 4.5g (32mmol) of anhydrous potassium carbonate, stir at room temperature for 15min, then raise the temperature to 45°C, and add 5.45g (20.4mmol) of N-( 3-bromopropyl) phthalimide, reacted at 45°C for 12h. After the reaction was completed, the solvent was evaporated under reduced pressure, and the residue was first extracted with dichloromethane, a...
Embodiment 2
[0045] 6-(2-4H-1-benzopyran-4-one)-{2-[3-(3-aminopropyl)-aminopropyl]1H-benzoisoquinoline-1,3(2H )-keto} dihydrochloride (18b) preparation:
[0046]
[0047] In addition to replacing N,N-dimethylethylenediamine with 3a in step (8), add 2mL of 4MHCl ethanol dropwise (V 4M盐酸 :V 乙醇 =1:2) solution, other synthesis and purification methods are the same as in Example 1. Yield: 86%, 1 HNMR (400MHz,D 2 O)δ: 8.15~8.26(m, 3H, Ar-H); 7.82(d, 1H, J=8.8Hz, Ar-H); 7.63~7.67(m, 1H, Ar-H); 7.51~7.56( m, 2H, Ar-H); 7.29(t, J=7.78Hz, 1H, Ar-H), 7.12(d, J=4.28Hz, 1H, Ar-H); 6.26(s, 1H, Ar-H ); 4.18(t, J=6.96Hz, 2H, 1×N-CH 2 ); 3.21~3.26(m,4H,2×N-CH 2 ); 3.15(t, J=7.82Hz, 2H, 1×N-CH 2 ); 2.12~2.20(m,4H,2×CH 2 ); ESI-MSm / z: 456.2[M+1–2HCl] + .Anal.calcdforC 27 h 27 Cl 2 N 3 o 4 1.85H 2 O: C57.73%, H5.51%, N7.48%; found C57.64%, H5.50%, N7.42%.
Embodiment 3
[0049] 6-(2-4H-1-benzopyran-4-one)-{2-[3-(4-aminobutyl)-aminopropyl]1H-benzoisoquinoline-1,3(2H )-keto} dihydrochloride (18c) preparation:
[0050]
[0051] In addition to replacing N,N-dimethylethylenediamine with 3b in step (8), add 2 mL of 4M HCl ethanol solution (V 4M盐酸 :V 乙醇 =1:2), other synthesis and purification methods are the same as in Example 1. Yield: 86%, white solid 1 HNMR (400MHz,D 2 O)δ: 7.74~7.78(m, 3H, Ar-H); 7.42(t, 1H, J=3.70Hz, Ar-H); 7.26~7.29(m, 2H, Ar-H); 7.05(s, 2H, Ar-H); 6.74(t, J=4.08Hz, 1H, Ar-H), 5.64(d, 1H, J=4.68Hz, Ar-H); 3.98(t, J=6.78Hz, 2H, 1×N-CH 2 ); 3.16~3.22(m,4H,2×N-CH 2 ); 3.10(t,2H,J=7.12Hz1×N-CH 2 ); 2.06(t,2H,J=7.02Hz1×CH 2 ); 1.78-1.89 (4H,2×CH 2 ).ESI-MIm / z:470.21[M+H-2HCl] + .Anal.calcdforC 28 h 29 Cl 2 N 3 o 4 1.6H 2 O: C58.87%, H5.68%, N7.36%; found C58.97%, H5.57%, N7.45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com