Parasite prevention and/or treatment pharmaceutical composition and preparation method and application thereof
A composition and parasite technology, applied in the pharmaceutical composition for preventing and/or treating pet parasitic diseases, the application field of medicine, can solve problems such as skin ulceration, animal injury, hair follicle damage, etc., and achieve smooth fur. Slippery, high safety, and the effect of promoting repair and healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
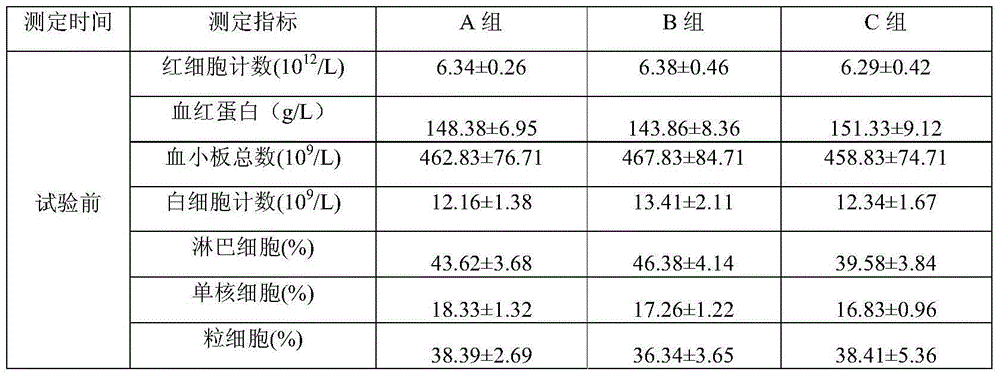
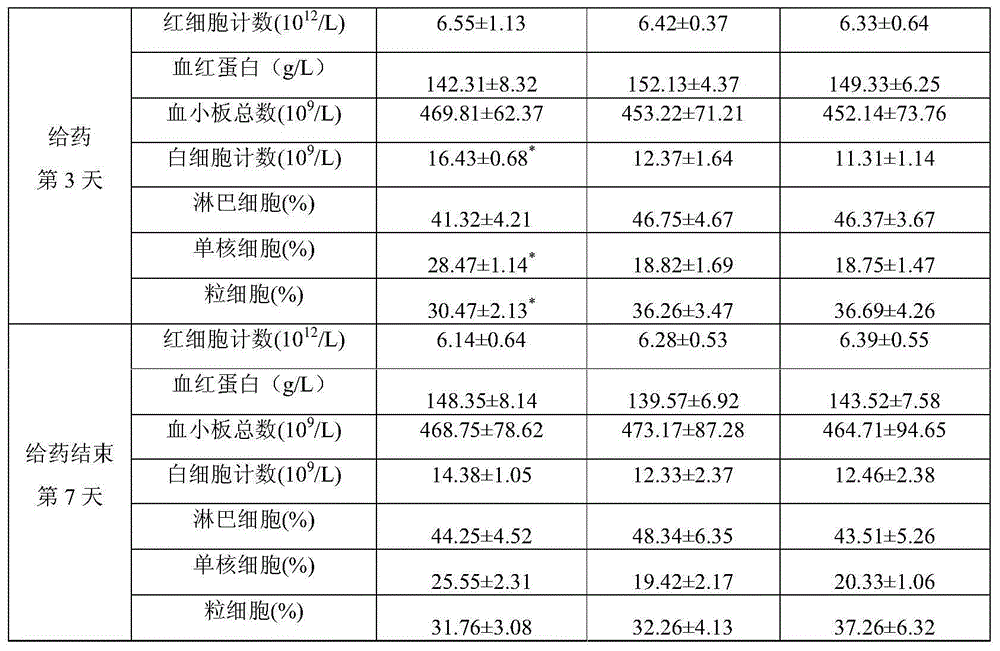
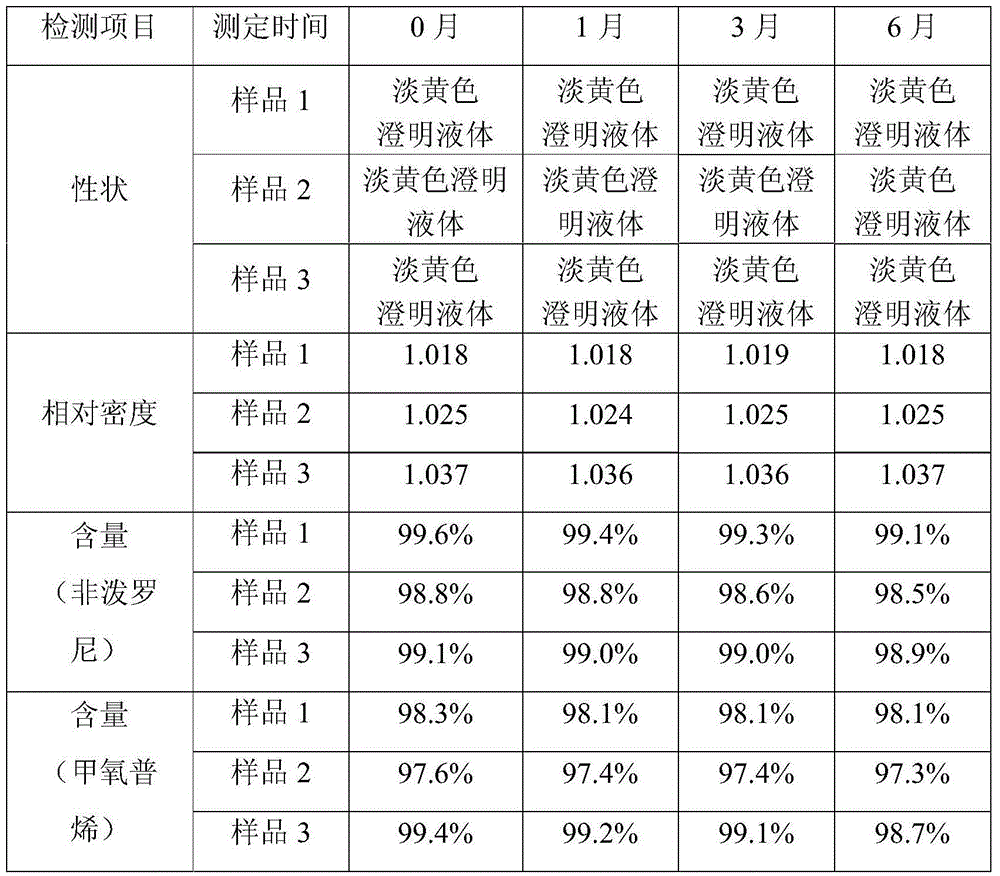
[0035] Embodiment 1: Preparation Example 1
[0036] Prescription: 5% fiprenil, 4% methoprene, 2% acetylated lanolin, tween-805%, 10% ethanol, 5% purified water and the balance of diethylene glycol monoethyl ether.
[0037] Preparation method: Weigh 5.211g of fiprenil and 2.033g of acetylated lanolin, put them in a clean container, add 50ml of diethylene glycol monoethyl ether, and stir to dissolve. Add 4.507g of methoprene and 5.137g of Tween-805.137g, and stir to dissolve. Add 5ml of purified water, add diethylenedimonoethyl ether to 100ml, stir evenly, and package to obtain a light yellow clear liquid with a relative density of 1.018.
Embodiment 2
[0038] Embodiment 2: Preparation Example 2
[0039] Prescription: 10% fiprenil, 9% methoprene, 3% lanolin, Tween-805%, 10% ethanol, 5% purified water and the balance of diethylene glycol monoethyl ether.
[0040] Preparation method: Weigh 10.265g of fiprenil and 3.051g of lanolin alcohol, put them in a clean container, add 50ml of diethylene glycol monoethyl ether, and stir to dissolve. Add 9.035g of methoprene and 805.124g of Tween, and stir to dissolve. Add 5ml of purified water, add diethylenedimonoethyl ether to 100ml, stir evenly, and package to obtain a light yellow clear liquid with a relative density of 1.025.
Embodiment 3
[0041] Embodiment 3: Preparation Example 3
[0042] Prescription: 20% of fiprenil, 18% of methoprene, 4% of acetylated lanolin, 805% of Tween, 10% of ethanol, 5% of purified water and the balance of diethylene glycol monoethyl ether.
[0043] Preparation method: Weigh 20.158g of fiprenil and 4.133g of lanolin alcohol, put them in a clean container, add 30ml of diethylene glycol monoethyl ether, and stir to dissolve. Add 18.365 g of methoprene and 805.036 g of Tween, and stir to dissolve. Add 5ml of purified water, add diethylenedimonoethyl ether to 100ml, stir evenly, and package to obtain a light yellow clear liquid with a relative density of 1.037.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com