Two-photon fluorescence probe and application thereof in detecting anoxic-zone nitroreductase
A two-photon fluorescence, anoxic region technology, applied in the field of fluorescent probes and organic small molecule fluorescent probes, can solve the problems that have not been widely reported and the two-photon fluorescent probes of nitroreductase are scarce.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis process of the two-photon fluorescent probe is as follows:
[0034] 1) Synthesis of compound 6-(4-hydroxyloxy)-2,3,4,4a-tetrahydro-xanthene-1-one (3):
[0035] In a 100mL round bottom flask, add 10mmol of 2,4-dihydroxybenzaldehyde (1), 15mmol of cyclohexenone (2) and 25mmol of triethylenediamine, then add water / 1,4-dioxane mixed solvent 60mL (V / V=1:1), under nitrogen atmosphere, heated to reflux for two days, distilled off the solvent under reduced pressure, and separated the residue by column chromatography to obtain light yellow solid 6-(4-hydroxyloxy)-2, 3,4,4a-tetrahydro-xanthene-1-one (3), referred to as GCTPOC. Yield: 15%.
[0036] 1 HNMR (400MHz, DMSO-d 6 ):10.17(s,1H),7.34(d,J=2.0Hz,1H),7.34(d,J=8.4Hz,1H),6.41(dd,J 1 =8.0Hz,J 2 =2.0Hz,1H),6.29(d,J=2.4Hz,1H),4.94(m,1H),2.36(m,3H),1.91(m,2H),1.67(m,1H);HR-MS calculated for C 13 h 12 o 3 [M-H] - m / z215.0703,found215.0747.
[0037] Synthesis of compound 6-((4-nitrobenzyl)oxy)-2,3,4,4a-tetrah...
Embodiment 2
[0043] Absorption Spectrum Changes of Compound GCTPOC-HY Two-Photon Hypoxic Fluorescent Probe
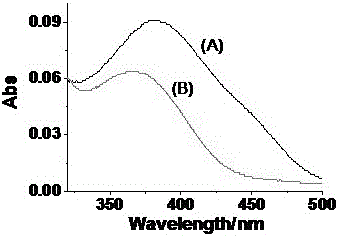
[0044] Prepare 1 part of 5mL 1mM dimethyl sulfoxide (DMSO) solution of the two-photon fluorescent probe GCTPOC-HY prepared by the present invention in advance, then take 25 μL and add them to two identical 5mL volumetric flasks, add 225 μL of DMSO solution, and then Both added nicotinamide adenine dinucleotide (NADH) 500μM. Nitroreductase phosphate buffer solution (10 μg / mL, 500 μL) was added to one of them, and both volumetric flasks were adjusted to 5 mL with phosphate buffer solution (PBS, pH=7.4), and then acted at 37°C for 50 minutes, and then the absorption spectrum test was performed. The absorption of a group of compounds with nitroreductase becomes stronger, the results are shown in figure 1 , where (A) is the absorption curve of the probe reacting with 1.0 μg / mL nitroreductase for 50 min; (B) is the absorption curve of the probe itself.
Embodiment 3
[0046] The Selectivity of Compound GCTPOC-HY Two-Photon Hypoxic Fluorescent Probe to Different Molecules or Ions
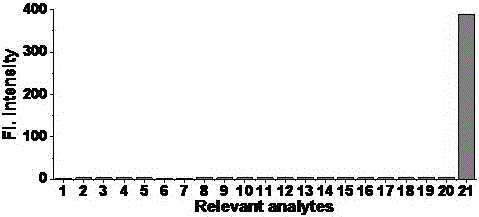
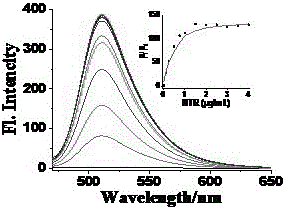
[0047] Prepare 5mL of PBS aqueous solution of various conventional ions and amino acids with a concentration of 40mM and a two-photon fluorescent probe with a concentration of 1mM as the mother solution; Dilute the volume of phosphate buffer to 5mL, shake well and perform fluorescence detection (λ ex =450nm,λ em =511nm), establish the curve of fluorescence intensity and each ion (or amino acid) (results see figure 2 ). After 50min, detect the change of the fluorescence emission spectrum of the solution, by figure 2 It can be found that other ions (or amino acids) have almost no effect on the fluorescence of compound GCTPOC-HY, but the addition of nitroreductase can significantly enhance the fluorescence of compound GCTPOC-HY. figure 2 Among them, the ions added in 1-21 are: probe, Na 2 SO 3 、NaNO 2 、NaNO 3 , NaClO, CaCl 2 、Na 2 S, FeSO 4 , KBr, H 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com