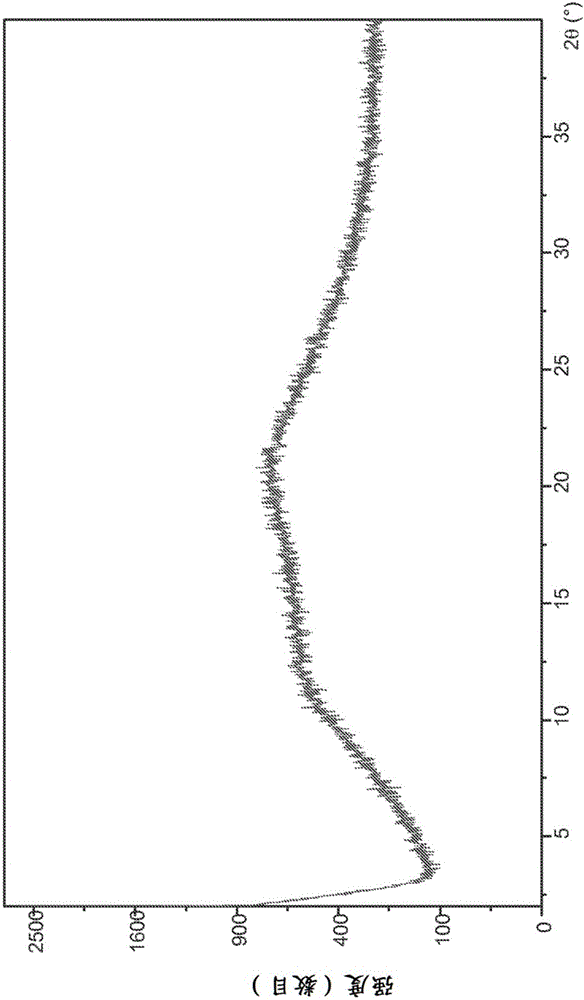
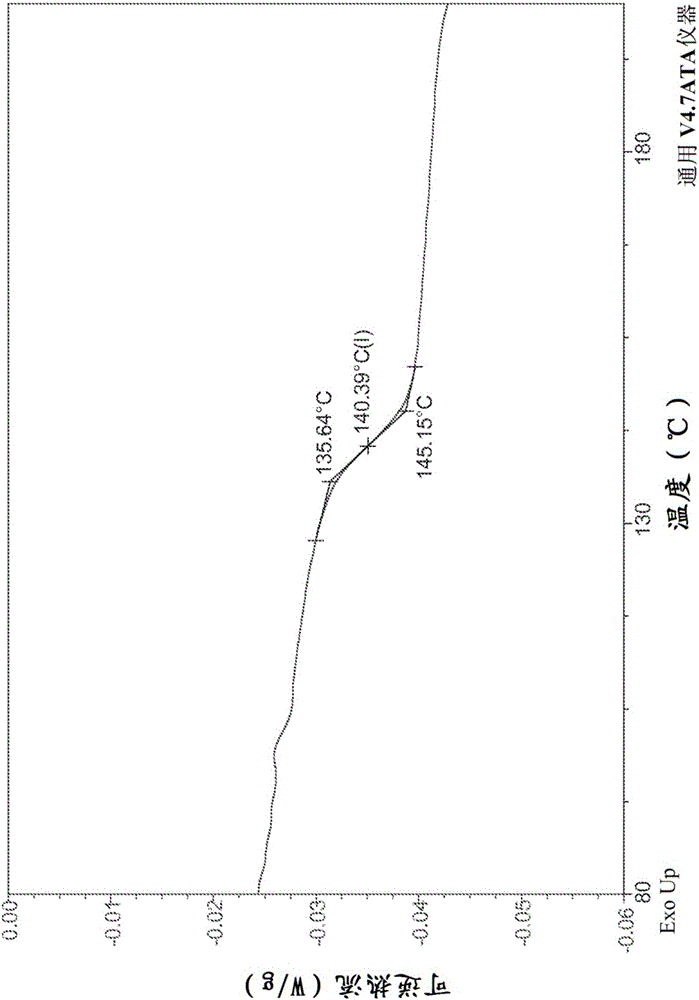
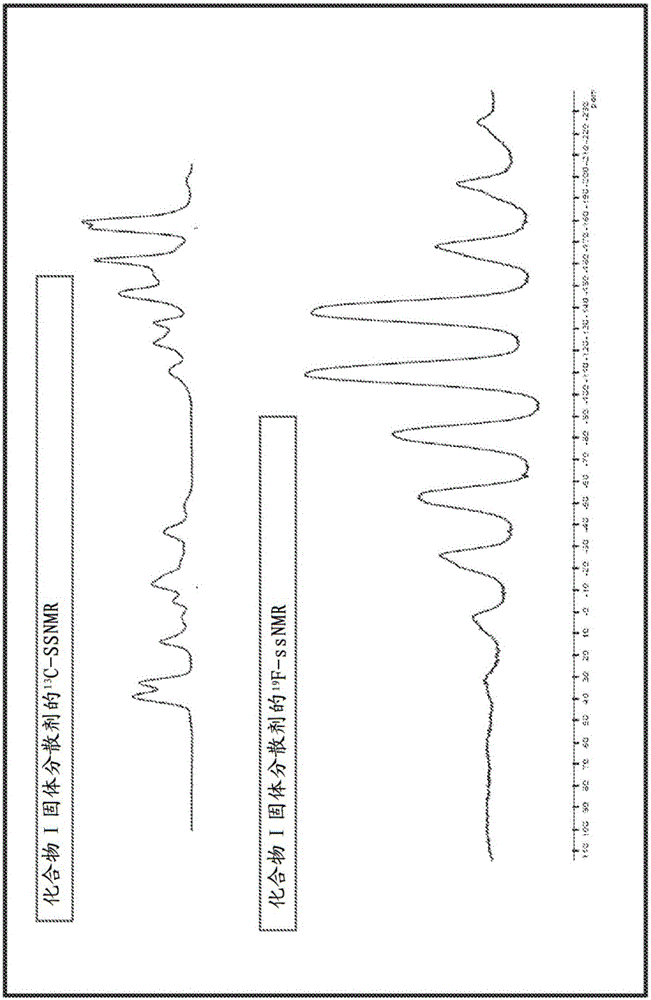
Combination formulation of two antiviral compounds
A combined use and composition technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as restricted use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0237] Embodiment 1: the synthesis of amorphous Ledipasvir
[0238] Methods for preparing various forms of ledipasvir can be found in US Publication Nos. 2013 / 0324740 and 2013 / 0324496. Both applications are incorporated herein by reference. The following is the procedure for isolating ledipasvir amorphous free base.
[0239] Combine ledipasvir acetone solvate (191.4 g) and acetonitrile (1356 g) in a reaction vessel and mix the contents until a solution forms. The ledipasvir in acetonitrile solution was slowly added to another reaction vessel containing vigorously stirred water (7870 g). The contents were stirred at about 23°C for about 30 minutes. The contents were filtered and dried at approximately 40-45° C. until constant weight was reached to give ledipasvir as an amorphous solid (146.4 g, 82% yield).
Embodiment 2
[0240] Example 2: Preparation and formulation of tablets
[0241] A. Dose Selection of Tablets
[0242] i. Sofosbuvir
[0243] The dosage of sofosbuvir chosen for the tablet is 400mg once daily. E. max The PK / PD model and early virology and human exposure data support the dose of 400mg sofosbuvir, and other trials also support the dose selection of 400mg sofosbuvir.
[0244] Average AUC of major metabolites of sofosbuvir at 400mg sofosbuvir dose tau This value is approximately 77% of the maximum change from baseline in HCV RNA measured according to this model, which is the plateau apex of the exposure-response sigmoid curve. In S shape E max In the model, there was a relatively linear exposure-response relationship during the 20% to 80% maximum effect range. Therefore, given that sofosbuvir exposure from the 200 mg tablet was dose proportional to single doses up to 1200 mg, doses below 400 mg would be expected to exhibit a considerable reduction in the magnitude of chang...
Embodiment 3
[0280] Example 3: PK, Stability and Dissolution Properties of Ledipasvir Single Drug Tablets and Ledipasvir / Sofosbuvir Tablets and Reduction of Food Effect and Gastric Acid Suppressant Effect
[0281] A. Bioavailability of Ledipasvir Single Drug Tablets
[0282] A series of in vivo experiments were performed to evaluate the potential benefits of this solid dispersion approach compared to conventional formulations, and the solid dispersion was optimized by identifying the optimal polymer type and relative polymer concentration within the dispersion.
[0283] In a canine model pretreated with pentagastrin, formulations containing free base amorphous form (4% w / w, 10 mg amorphous free base tablet) and formulations containing ledipasvirD-tartrate (5.85% w / w, 10mgD-tartrate tablets) have equivalent bioavailability, wherein these two preparations are conventional preparations, see Table 7 for the results. Gogastrin is a synthetic polypeptide that stimulates the secretion of gastric...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com