A double-copy human p53 gene recombinant adenovirus and its preparation method
A technology of p53 gene and recombinant adenovirus, which is applied in the field of biomedicine, can solve problems such as side effects, and achieve the effects of strong specificity, increased gene expression, and reduced virus usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
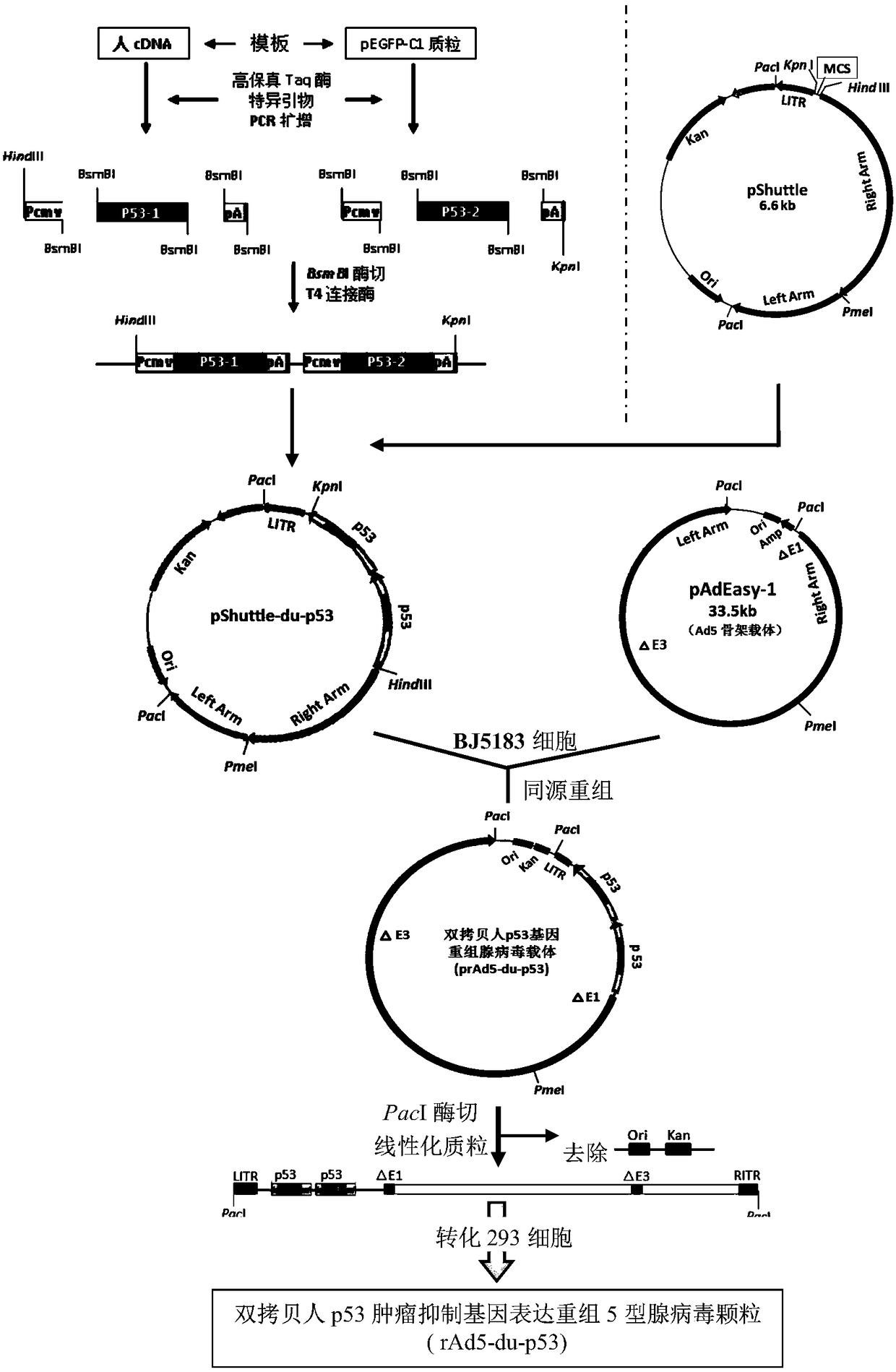
[0041] 1. Construction of double-copy human p53 tumor suppressor gene eukaryotic expression cassette:
[0042] Apply the golden gate cloning technology (goldengate cloning) that is used for one-time assembly of multiple molecular fragments in the new molecular cloning technology, design and synthesize the double-copy p53 gene expression cassette, that is, include the eukaryotic cell expression promoter (used in the embodiment of the present invention Developing reading frame (ORF) and polyadenylation tail (poly A) for cytomegalovirus (CMV promoter), p53 tumor suppressor gene: construction of human cytomegalovirus (CMV) virus early promoter--Kozak sequence-P53 Gene--3' end non-coding sequence polyadenylation tail (poly A) sequence-human cytomegalovirus (CMV) virus early promoter--Kozak sequence--P53 gene--3' end non-coding sequence polyadenylation Nucleotide tail (poly A) sequence.
[0043] The Golden Gate cloning technique utilizes type IIS restriction endonuclease, whose DNA...
Embodiment 2
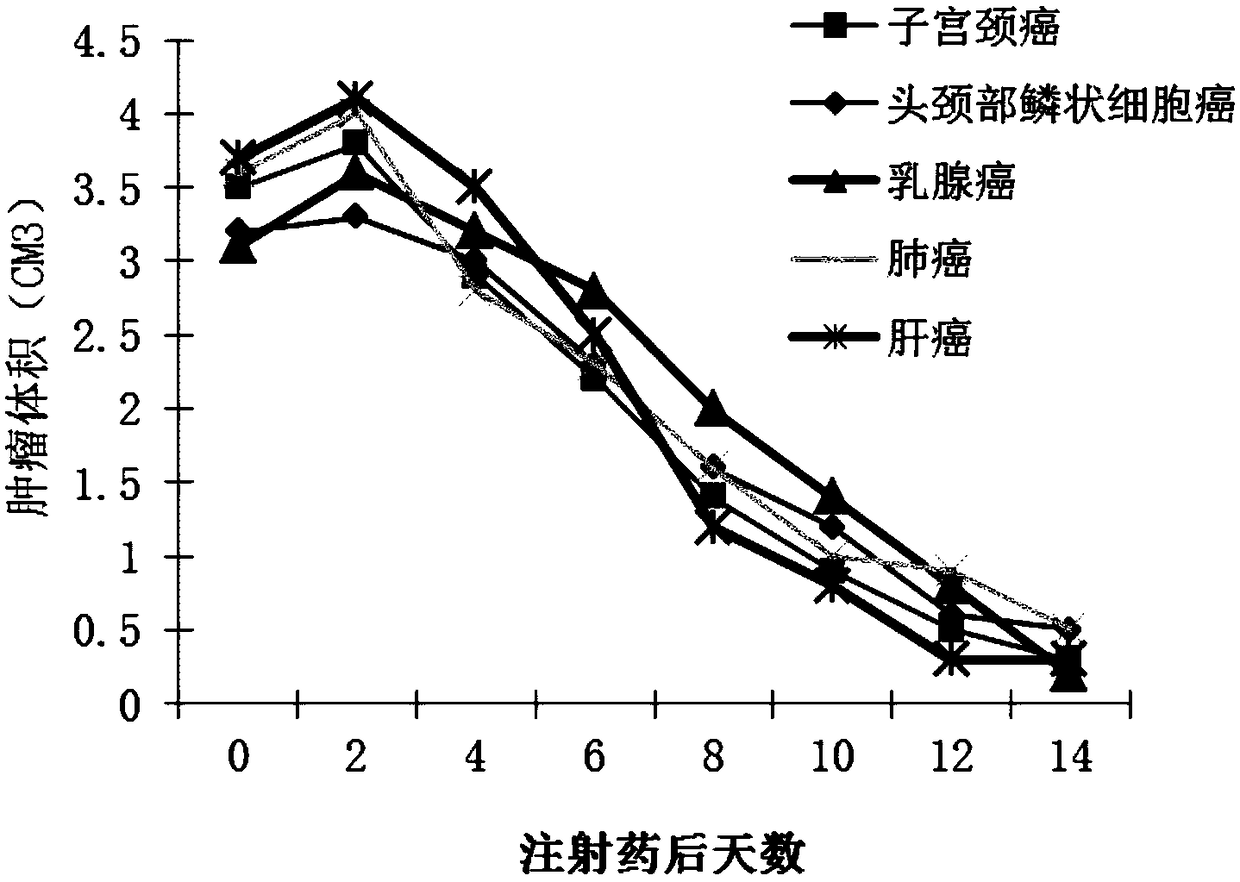
[0053] (1) Double plaque formation experiment and determination of virus particle content
[0054] Use a series of dilutions of purified virus (double-copy human p53 gene recombinant adenovirus) to infect monolayer HEK293 cells, and serially serially dilute the purified virus at a ratio of 1:10, and select 10 6 to 10 13Dilute virus (double-copy human p53 gene recombinant adenovirus) suspensions were added to dense single-layer HEK293 cell culture flasks to allow the virus to adsorb, and cultured at 37°C. After 1 hour, the virus suspension was sucked away, and then covered with a layer to melt agar, cultured at 37°C. At least 2 copies of each dilution gradient. After 24 hours, stain with neutral red and check the results. After the virus (recombinant adenovirus with double-copy human p53 gene) replicates in HEK293 cells, it can produce a limited infection focus, namely plaque. Live cells were stained with neutral red, and unstained plaques could be seen, and the virus conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com