Cancer models and associated methods
A technology of cancer and models, applied in the field of prevention or treatment of diseases, disorders or diseases or their symptoms, capable of solving problems such as human debility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0144] Example 4 illustrates the effect on body weight of mice initially measured prior to injection of the indicated transgenes and subsequently determined at 3, 5 and 23 weeks post-injection. Such as Figure 4 Depicted in , transgenic db / db mice co-expressing the FGF19 variant M70 and FGF19 exhibited significant body weight loss compared to control-dosed animals, whereas that observed in mice expressing only the FGF19 transgene The effect on body weight was not significant. The body weight changes observed in mice co-expressing the FGF19 and M70 transgenes were also reflected in reduced liver weights compared to liver weights harvested from animals in the control group.
[0145] The effect of transgene expression on blood glucose was also assessed before transgene injection and at 3, 5, and 23 weeks post-injection in a manner similar to body weight measurements. Figure 5 The results presented in demonstrate that transgenic db / db mice co-expressing the FGF19 variant M70 an...
Embodiment 2
[0304] Materials and methods for Examples 2-5
[0305] The following methods and materials were used for Examples 2-5.
[0306] animal .Under controlled light (12 hours light and 12 hours dark cycle, 6:30p.m.-6:30a.m. dark), temperature (22±4℃) and humidity (50%±20%) , db / db mice (The Jackson Laboratory, Bar Harbor, ME) (approximately 15-week-old mice and approximately 36-48 g body weight at the start of treatment) were cared for according to welfare guidelines. Mice had free access to autoclaved distilled water and ad libitum access to a commercial diet (Harlan Laboratories, Indianapolis, IN, Teklad Global 18% Protein Rodent Diet) containing 1 kcal % fat, 24 kcal % protein and 58 kcal % carbohydrates. All animal studies were approved by the NGM Institutional Animal Care and Use Committee (NGM Institutional Animal Care and Use Committee).
[0307] Nucleic Acid and Amino Acid Sequences . FGF19 ORF (ORF encoding cDNA of hFGF19 (GenBank Accession No. NM_005117.2) and its en...
Embodiment 2
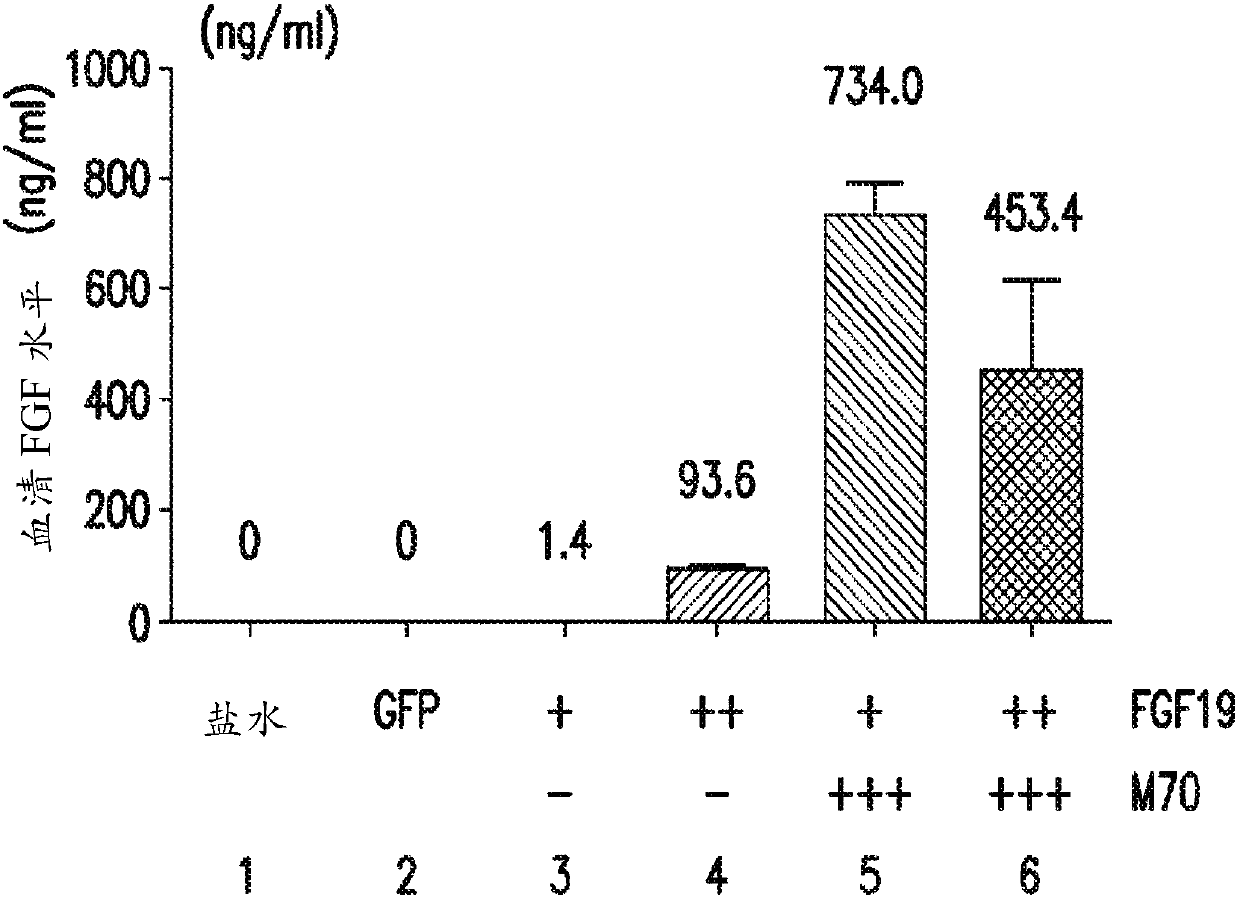
[0317] Plasma FGF19 levels in db / db mice after gene delivery
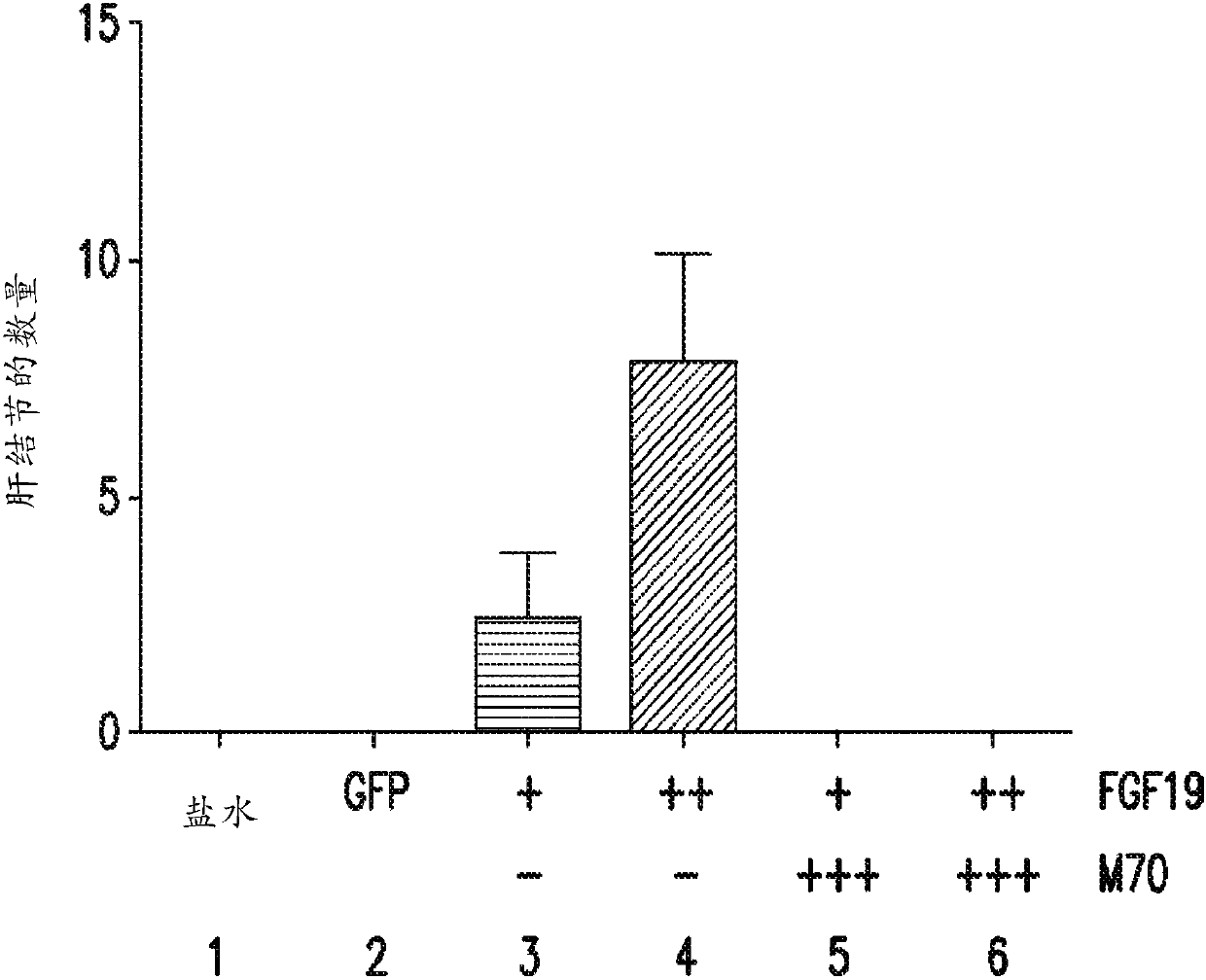
[0318] A 24-week study was performed to assess whether the FGF19 variant M70 could prevent FGF19-induced tumorigenesis in db / db mice. As an alternative to conventional delivery methods, AAV was used as a vehicle in this example (and subsequent examples 2-4) to deliver and express relevant exogenous genes in mice and achieve continuous, sustained, and systemic exposure to The proteins encoded by these transgenes.
[0319] Prior to gene delivery, mice were divided into six groups (5 male mice / group) as described in Table 1, and blood glucose and body weight measurements were recorded for each mouse.
[0320] Table 1
[0321]
[0322] At week 0, mice were injected with 0.2 mL of saline or 0.2 mL of one of the AAV constructs from groups 2-6. At weeks 3 and 5, blood glucose and body weight measurements were again recorded for each mouse in groups 1-6.
[0323] Five weeks after gene delivery, FGF19 concentrations ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com