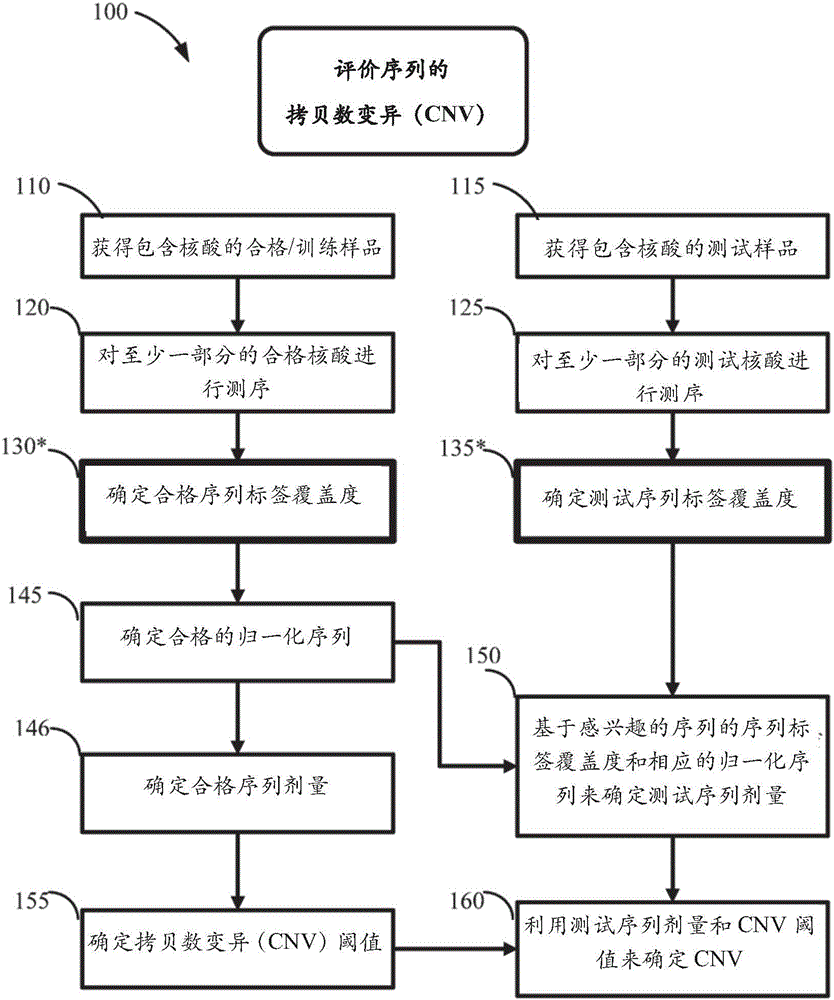
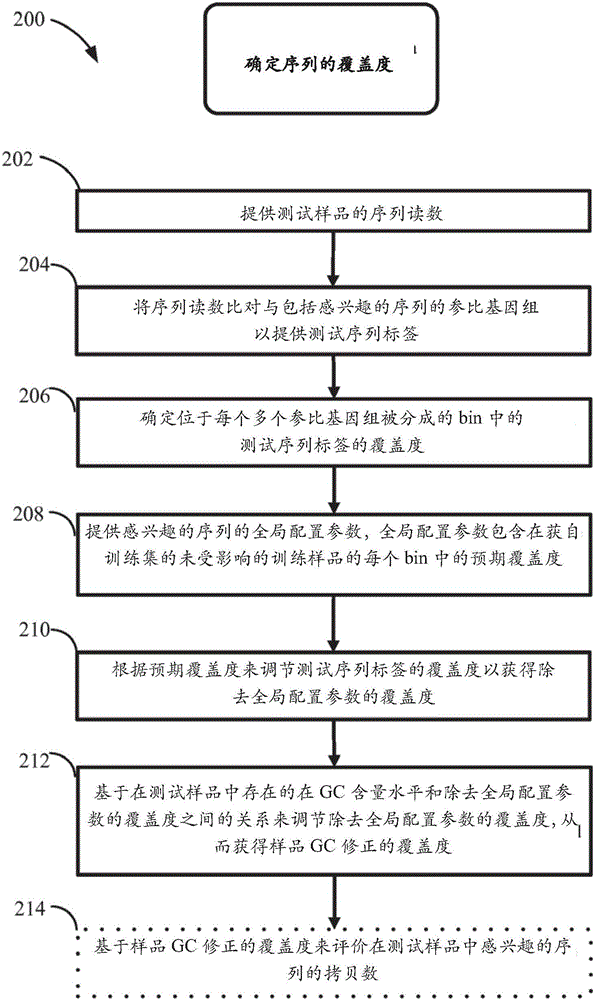
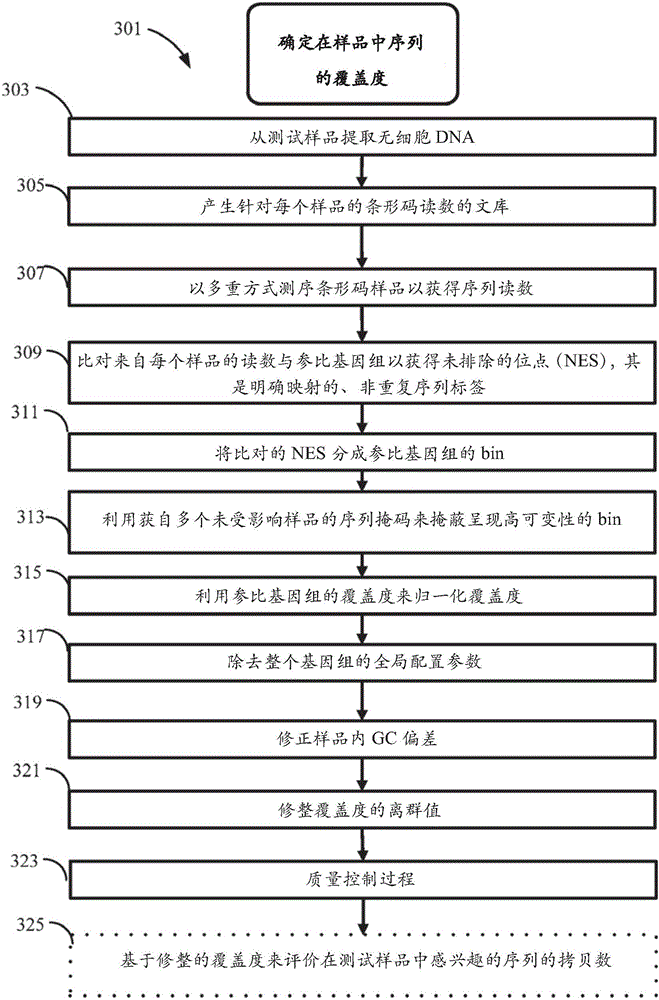
Method for improving the sensitivity of detection in determining copy number variations
A technology of copy number and readout, applied in biochemical equipment and methods, microbiological determination/inspection, electrical digital data processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0302] Sequencing library preparation
[0303] In one embodiment, the methods described herein can utilize next-generation sequencing (NGS), which allows multiple samples to be sequenced individually in a single sequencing run, either as genomic molecules (i.e., single-plex sequencing) or as genomic molecules containing indexes. pooled samples (eg, multiplexed sequencing). These methods can generate DNA sequences up to hundreds of millions of reads. In various embodiments, the sequence of genomic nucleic acid, and / or indexed genomic nucleic acid, can be determined using, for example, next generation sequencing (NGS) techniques described herein. In various embodiments, one or more processors as described herein can be utilized to analyze large amounts of sequence data obtained using NGS.
[0304] In various embodiments, the application of the sequencing techniques described above does not involve the preparation of sequencing libraries.
[0305] However, in certain embodim...
Embodiment 1
[0442] Preparation and sequencing of raw and enriched sequencing libraries
[0443] a. Preparation of sequencing library - simplified method (ABB)
[0444] All sequencing libraries, ie, raw and enriched libraries, were prepared from approximately 2 ng of purified cfDNA extracted from maternal plasma. Take advantage of NEBNext TM The reagent of DNASamplePrepDNAReagentSet1 (product number E6000L; NewEnglandBiolabs, Ipswich, MA) is used for library preparation, for as follows. Since cell-free plasma DNA is fragmented in nature, plasma DNA samples were not further fragmented by nebulization or sonication. according to EndRepairModule, at 20°C, by DNA polymerization in a 1.5 ml microcentrifuge tube with the aid of 5 μl of 10X phosphorylation buffer, 2 μl of deoxynucleotide solution mixture (10 mM for each dNTP), 1 μl of a 1:5 dilution Enzyme I, 1 μl of T4 DNA polymerase and 1 μl of T4 polynucleotide kinase, available at NEBNext TM In DNASamplePrepDNAReagentSet1, cfDNA w...
Embodiment 2
[0454] Accurate Aneuploidy Detection in Twin Pregnancies
[0455] introduction
[0456] Noninvasive prenatal testing (NIPT) of total cell-free DNA (cfDNA) using whole-genome massively parallel sequencing has been shown to be a very accurate and reliable method for detecting fetal chromosomal aneuploidy. 参见,BianchiDW,PlattLD,GoldbergJD,等人Genome-WidefetalaneuploidydetectionbymaternalplasmaDNAsequencing.ObstetGynecol2012;119:890-901;FanHC,BlumenfeldYJ,ChitkaraU,HudginsL,QuakeSR.NoninvasivediagnosisoffetalaneuploidybyshotgunsequencingDNAfrommaternalblood.ProcNatlAcadSciUSA2008;105:16266-71;SehnertAJ,RhecsB,ComstockD,等人 Optimal detectionoffetalchromosomalabnormalitiesbymassivelyparallelDNAsequencingofcell-freefetalDNAfrommaternalblood. ClinChem2011;57:1042-9. The point-of-care test described above detects trisomies 21, 18, 13 and sex chromosome aneuploidy from a single maternal blood sample. The point-of-care tests described above are currently indicated for pregnant women with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com