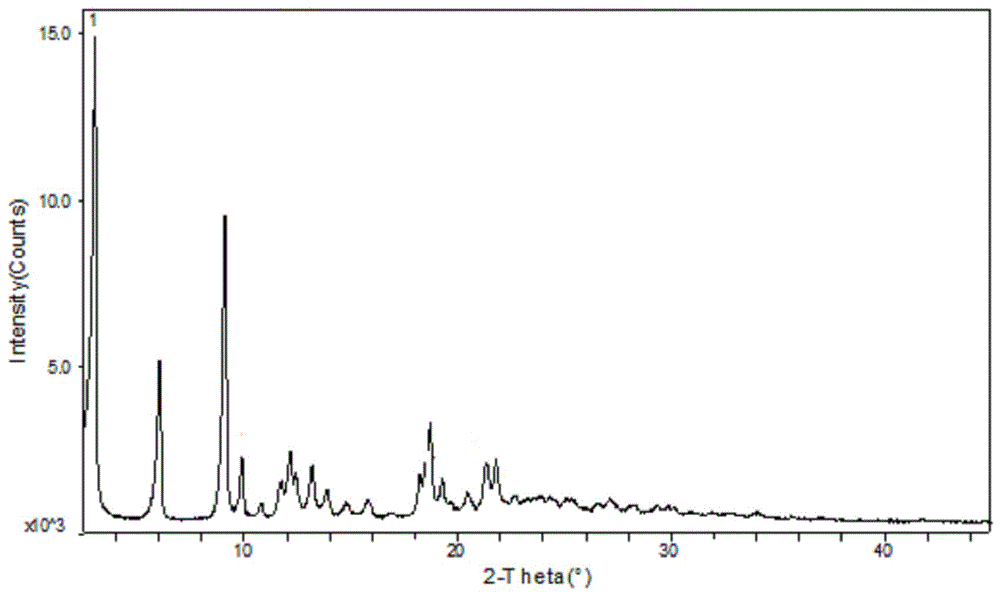
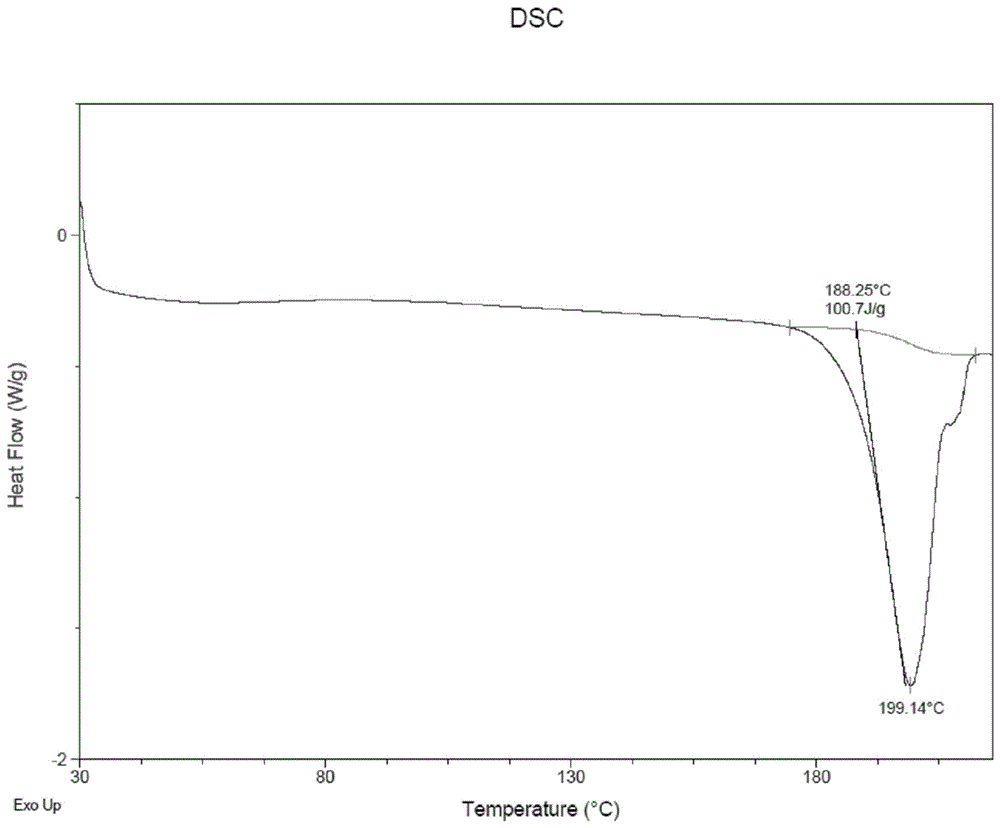
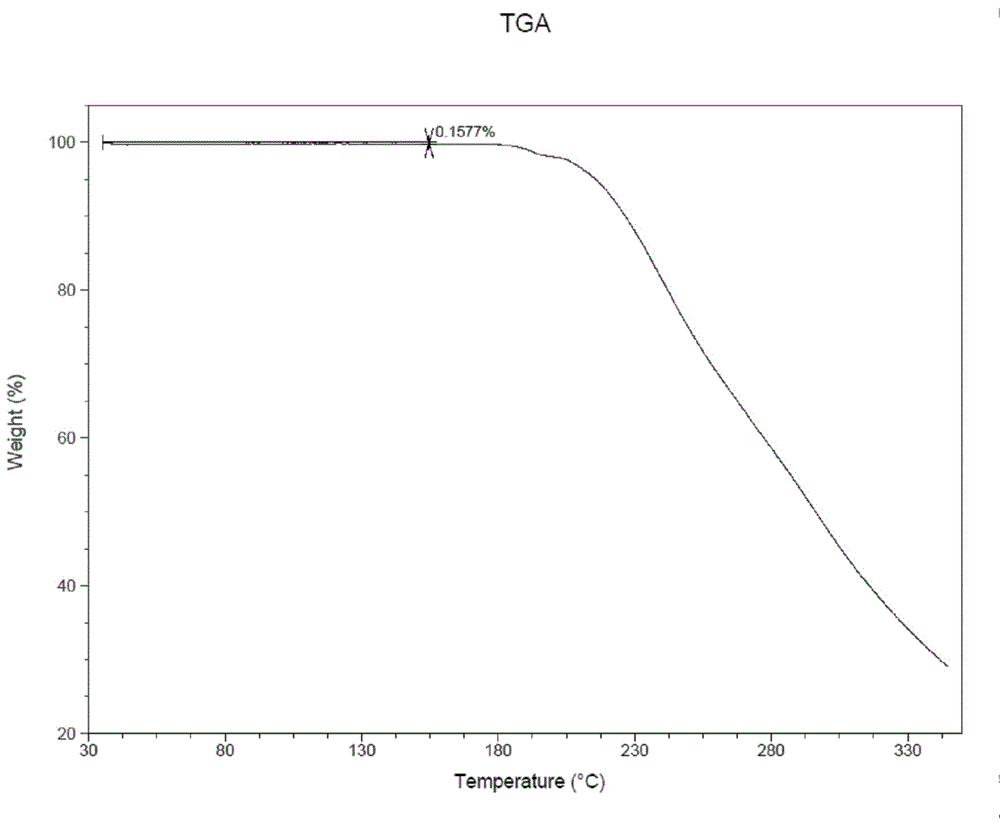
Method for preparing A-type crystal of Atazanavir disulfate
A technology of bisulfate and atazanavir is applied in the field of preparation of type A crystal of atazanavir bisulfate, which can solve the problems of harsh speed requirements, poor process stability and high purity requirements of concentrated sulfuric acid, and is convenient for industrial production. , The effect of high crystal purity and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of atazanavir bisulfate type A crystal comprises the steps:
[0044] a) Add the compound atazanavir free base (100.0g, 142mmol) and ethanol (750mL) into a three-necked flask (1000mL) at room temperature (25°C) and maintain the temperature at 25-30 o C stirred for 30 minutes, the reaction solution was a white or off-white suspension, and concentrated sulfuric acid (8.6mL, 0.05mL / s) was slowly added dropwise to the reaction solution, the solution gradually became clear, and the solution was heated to 35-40 o C, add atazanavir ethanolate type E crystal as seed crystal (4.26 mmol), stir for 15-20min, add 750ml of n-heptane (1 hour) and stir, cool to 15-25 o C. Stir the resulting crystalline mixture for 6-8 hours, collect the solid by filtration, wash the filter cake with ethanol / n-heptane=1:1 (10mL), and after drying, obtain about 102g of white or off-white powdery solid Azana Ethanol compound type E crystals, HPLC purity 99.90%, maximum single impu...
Embodiment 2
[0047] a) Add the compound atazanavir free base (100.0g, 142mmol) and ethanol (500mL) into a three-necked flask (1000mL) at room temperature (22°C) and maintain the temperature at 22-30 o C stirred for 30 minutes, the reaction solution was a white or off-white suspension, and concentrated sulfuric acid (8.3mL, 0.05mL / s) was slowly added dropwise to the reaction solution, the solution gradually became clear, and the solution was heated to 30-35 o C, add atazanavir ethanolate type E crystal as seed crystal (2.84 mmol), stir for 15-20min, add 600ml of n-heptane (1 hour) and stir, cool to 15-25 o C. Stir the resulting crystalline mixture for 6-8 hours, collect the solid by filtration, wash the filter cake with ethanol / n-heptane=1:1 (10mL), and after drying, obtain about 100g of white or off-white powdery solid Azana Ethanol compound E crystals. HPLC purity is 99.87%, the maximum single heterogeneity is 0.04%, and diastereomers and enantiomers are not detected; GC solvent residue...
Embodiment 3
[0050] a) Add the compound atazanavir free base (100.0g, 142mmol) and ethanol (2000mL) into a three-necked flask (5000mL) at room temperature (22°C) and maintain the temperature at 22-30 o C stirred for 30 minutes, the reaction solution was a white or off-white suspension, and concentrated sulfuric acid (8.8mL, 0.05mL / s) was slowly added dropwise to the reaction solution, the solution gradually became clear, and the solution was heated to 40-45 o C, add atazanavir ethanolate type E crystal as seed crystal (5.68 mmol), stir for 15-20min, add 1600ml of n-heptane (1 hour) and stir, cool to 15-25 o C. Stir the resulting crystalline mixture for 6-8 hours, collect the solid by filtration, wash the filter cake with ethanol / n-heptane=1:1 (10mL), and after drying, obtain about 100g of white or off-white powdery solid Azana Ethanol compound E crystals. The HPLC purity is 99.80%, the maximum single heterogeneity is 0.05%, and diastereomers and enantiomers are not detected; GC solvent r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com