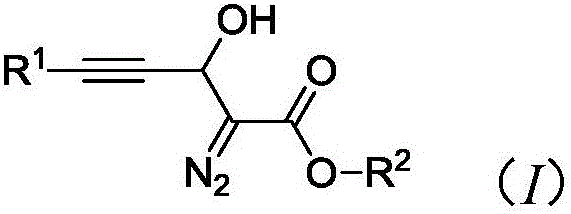
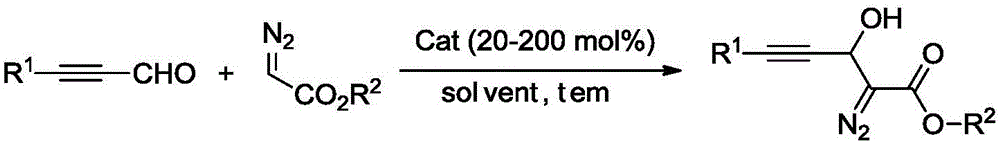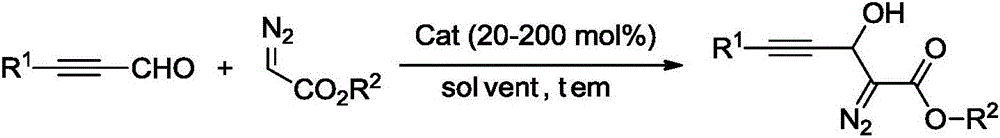Synthesis method of 3-hydroxyl diazoester intermediate alkyne compound
A technology of hydroxydiazo ester group and synthesis method, which is applied in the direction of organic chemistry, etc., and can solve problems such as corrosion and difficult operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0027] Synthesis of 3-Hydroxydiazoacetate Ethylphenylacetylene Compounds
[0028] Add 20 mol% K to the reaction vessel 2 CO 3 , 0.2mmol phenylpropynaldehyde, then add 1ml N,N-dimethylformamide, 0.2mmol ethyl diazoacetate, react at 25°C, after the reaction, wash with water, then extract with organic solvent, dry, and distill under reduced pressure The solvent was removed by concentration, and the crude product was separated by column chromatography to obtain the target product with a yield of 45%.
Synthetic example 2
[0030] Synthesis of 3-Hydroxydiazoacetate ethyl p-methylphenylacetylene compound
[0031] Add 50mol% triethylamine, 0.2mmol p-tolylpropynaldehyde to the reaction vessel, then add 1ml acetonitrile, 0.24mmol ethyl diazoacetate, and react at 40°C. After the reaction, wash with water, and then wash with organic solvent Extraction, drying, distillation and concentration under reduced pressure to remove the solvent, the crude product was separated by column chromatography to obtain the target product with a yield of 61%.
Synthetic example 3
[0033] Synthesis of 3-Hydroxydiazoacetate ethyl p-chlorophenylacetylene compound
[0034] Add 100 mol% CS to the reaction vessel 2 CO 3 , 0.2mmol p-bromophenylpropynaldehyde, then add 1ml 1,4-dioxane, 0.3mmol ethyl diazoacetate, react at 60°C, after the reaction, wash with water, then extract with organic solvent, dry, reduce Pressure distillation concentrated to remove the solvent, and the crude product was separated by column chromatography to obtain the target product with a yield of 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com