A kind of chiral sulfonamide derivative and its preparation method and application
A technology for sulfonamides and derivatives, applied in the field of chiral sulfonamide derivatives and their preparation, can solve the problems of unfavorable sulfonamide derivatives using industrial synthesis, non-recyclable chemical waste, low atom economy and the like , to achieve the effect of low cost, less waste and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The preparation method for synthesizing chiral sulfonamide derivatives of the present invention comprises sulfonamide, imine, rhodium acetate, The molecular sieve is dissolved in an organic solvent to prepare a mixed solution; the aryl diazoacetate is dissolved in an organic solvent to prepare a diazo compound solution; at -10°C, the diazo compound solution is added to the aforementioned mixed solution with a syringe pump; at the same time Vigorous stirring; after the dropwise addition of the diazo compound solution, continue stirring at -10°C for 60 minutes until the diazo compound is completely consumed; the crude product is subjected to column chromatography (with ethyl acetate: petroleum ether = 1:20~1:20) 10 is the eluent) to give pure product.
[0089] The synthetic reaction process is as follows:
[0090]
[0091] In the reaction formula (II),
[0092] R is aryl or alkyl, selected from phenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 4-methoxyphenyl,...
Embodiment 1
[0097]
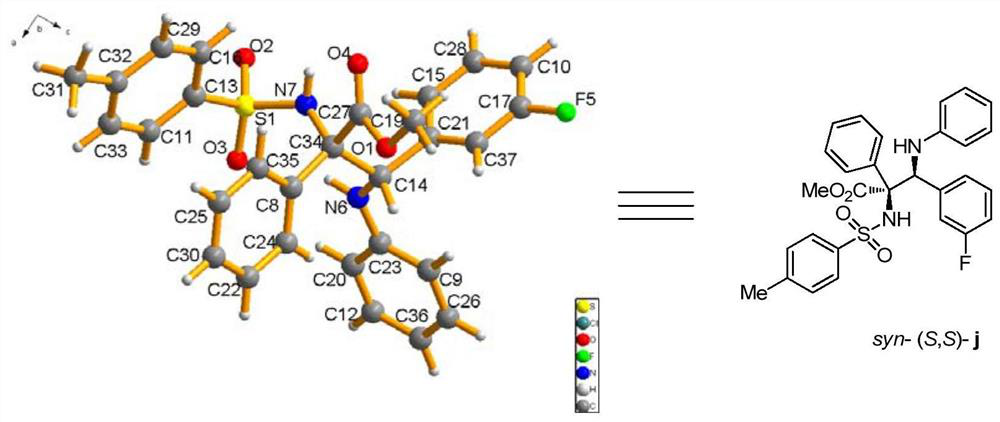
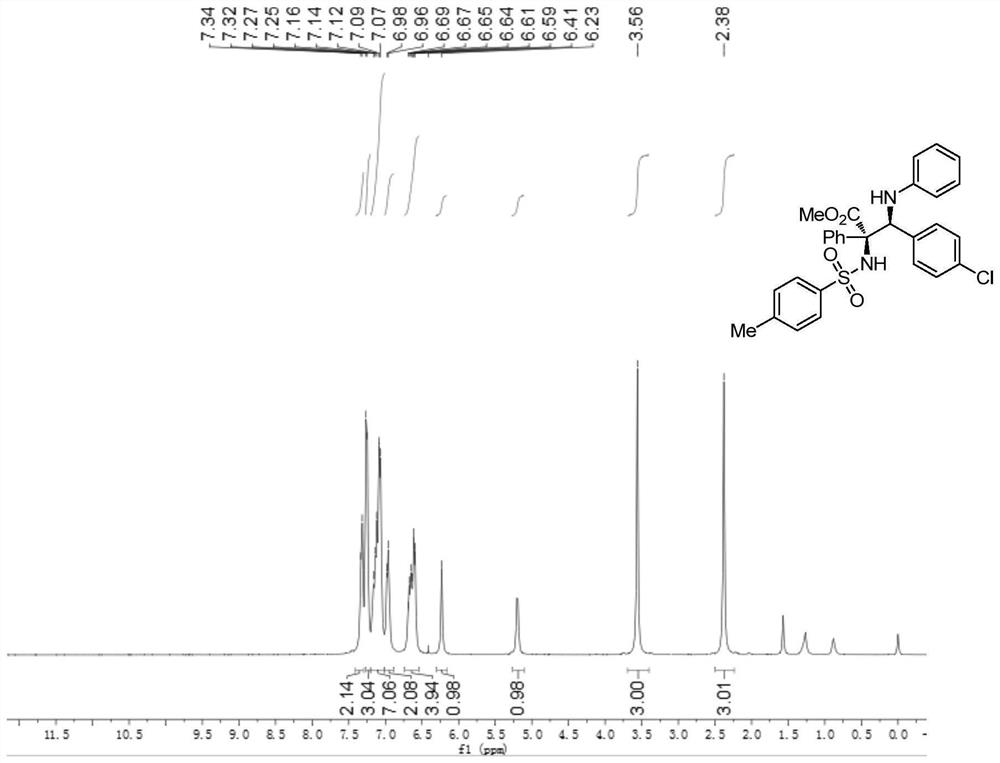
[0098] p-Toluenesulfonamide (0.36 mmol), p-chlorobenzylidene aniline (0.30 mmol), rhodium acetate (0.006 mmol), (R)-3,3'-bis(triphenylsilyl)binaphthol phosphate (0.03mmol), Molecular sieve (150 mg) was dissolved in 3.0 mL of anhydrous toluene to prepare mixed solution A; methyl phenyldiazoacetate was dissolved in 1.0 mL of anhydrous toluene to prepare diazo compound solution B; at -10°C, solution B was dissolved At -10°C, the mixed solution A was added with a syringe pump within 1 hour. After the injection of liquid B, the reaction system continued to stir at -10°C for 60 minutes. After the reaction is completed, filter to obtain the filtrate by rotary evaporation to remove the solvent, and then purify the crude product by column chromatography to obtain the pure product, which is a white solid. Its structure is shown in formula (a). The product was isolated in 83% yield, with a dr value greater than 20:1 and an ee value of 99%. of the product 1 The schematic ...
Embodiment 2
[0101]
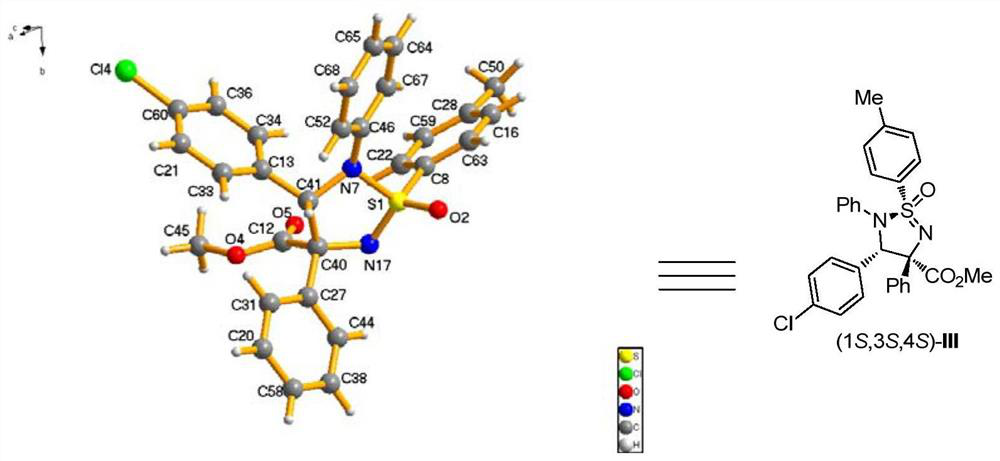
[0102] p-4-tert-Butylbenzenesulfonamide (0.36 mmol), p-chlorobenzylidene aniline (0.30 mmol), rhodium acetate (0.006 mmol), (R)-3,3'-bis(triphenylsilyl) Binaphthol phosphate (0.03mmol), Molecular sieve (150 mg) was dissolved in 3.0 mL of anhydrous toluene to prepare mixed solution A; methyl phenyldiazoacetate was dissolved in 1.0 mL of anhydrous toluene to prepare diazo compound solution B; at -10°C, solution B was dissolved At -10°C, the mixed solution A was added with a syringe pump within 1 hour. After the injection of liquid B, the reaction system continued to stir at -10°C for 60 minutes. After the reaction is completed, filter to obtain the filtrate by rotary evaporation to remove the solvent, and then purify the crude product by column chromatography to obtain the pure product, which is a white solid. Its structure is shown in formula (b). The product was isolated in 78% yield, with a dr value greater than 20:1 and an ee value of 90%. of the product 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com