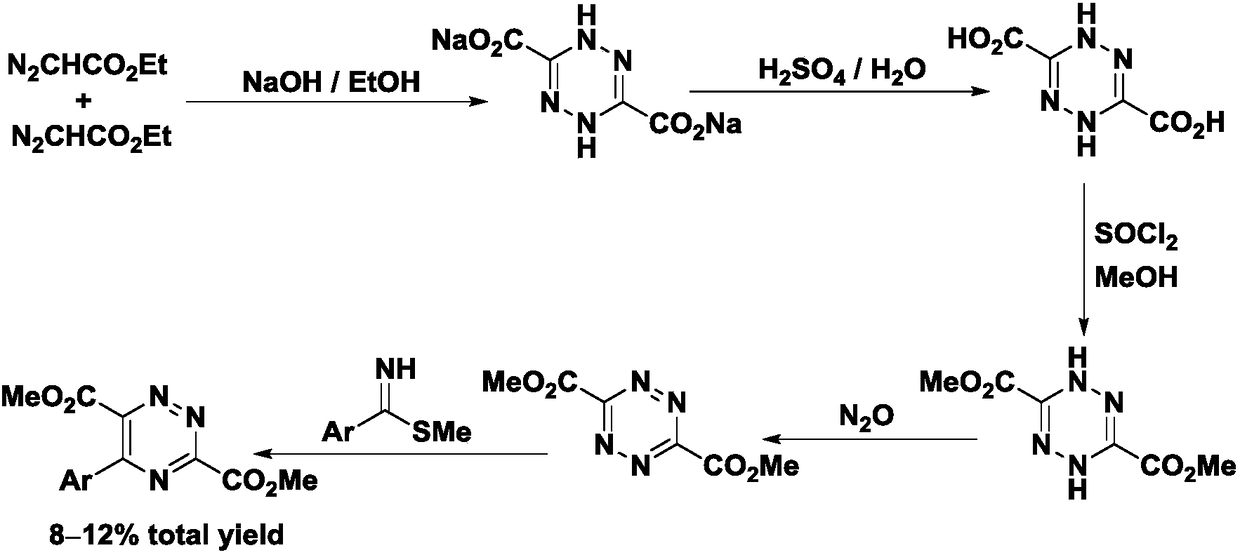
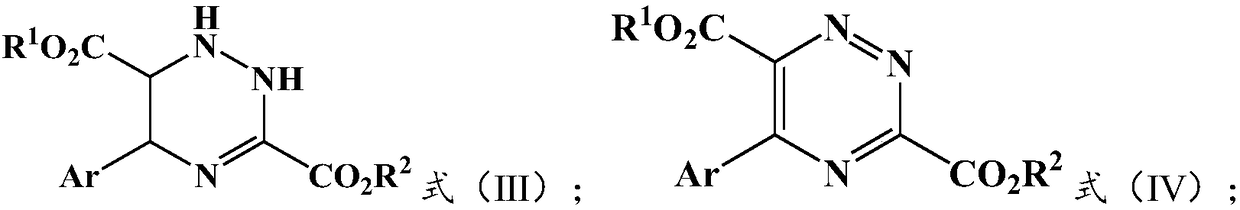Preparation method of 5-aryl-1,2,4-triazine-3,6-diformate and application thereof
A technology of diformate and triazine, applied in the field of chemical biology, can solve the problems of low total product yield, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
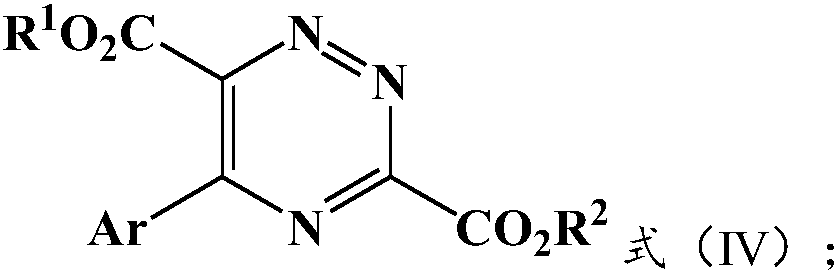
[0030] The present invention provides a kind of preparation method of 5-aryl-1,2,4-triazazine-3,6-dicarboxylate, comprising: S-1) ethyl diazo shown in formula (I) Ester and the glycine ester aromatic aldimine shown in formula (II) react under the condition that silver salt catalyst and inorganic base exist, obtain the intermediate shown in formula (III); S-2) described formula (III) ) is mixed with an oxidizing agent for oxidation reaction to obtain 5-aryl-1,2,4-triazazine-3,6-dicarboxylate described in formula (IV);
[0031] N 2 CHCO 2 R 1 Formula (I);
[0032]
[0033] Among them, R 1 with R 2 Each independently is a substituted or unsubstituted C1-C12 alkyl group, a substituted or unsubstituted C3-C8 cycloalkyl group, a substituted or unsubstituted C6-C20 aromatic group; preferably a substituted or unsubstituted C1-C8 C10 alkyl, substituted or unsubstituted C3-C6 cycloalkyl, substituted or unsubstituted C6-C15 aryl; more preferably substituted or unsubstituted C1-C...
Embodiment 1
[0053] Example 1: Methyl 5-phenyl-1,2,4-triazazine-3,6-dicarboxylate
[0054]
[0055] Step 1: Preparation of methyl 5-phenyl-1,2,5,6-tetrahydro-1,2,4-triazazine-3,6-dicarboxylate
[0056] In a dry Schlenk reaction flask (100 mL) was added glycine methyl benzaldimine (0.71 g, 4 mmol), AgOAc (67 mg, 0.4 mmol) and Cs 2 CO 3 (1.30g, 4mmol), 15mL of tetrahydrofuran was added to the system, and stirred at room temperature for 3 minutes; then a tetrahydrofuran solution of methyl diazoacetate (0.36mL, 4mmol) was added to the system, and stirred at 60°C for 24 hours. After the reaction was complete as detected by TLC, the solvent was removed under reduced pressure; then 40 mL of ethyl acetate and 20 mL of water were added to the system for liquid separation, and the aqueous phase was extracted with ethyl acetate (10 mL×3), and the organic phases were combined and washed with water (20 mL). twice, anhydrous MgSO 4 Drying, column chromatography (eluent: petroleum ether / ethyl aceta...
Embodiment 2
[0061] Example 2: Methyl 5-(4-methylphenyl)-1,2,4-triazazine-3,6-dicarboxylate
[0062]
[0063] Step 1: Preparation of methyl 5-(4-methylphenyl)-1,2,5,6-tetrahydro-1,2,4-triazazine-3,6-dicarboxylate
[0064] Add glycine methyl ester-(4-methylbenzaldehyde)imine (0.76g, 4mmol) to a dry Schlenk reaction flask (100mL), Ag 2 O (184mg, 0.8mmol) and K 2 CO 3 (1.10g, 8mmol), add 15mL of acetonitrile to the system, stir at room temperature for 3 minutes; then add a solution of methyl diazoacetate in acetonitrile (0.36mL, 4mmol), and stir at 40°C for 30 hours. After the reaction was complete as detected by TLC, the solvent was removed under reduced pressure; then 40 mL of ethyl acetate and 20 mL of water were added to the system for liquid separation, and the aqueous phase was extracted with ethyl acetate (10 mL×3), and the organic phases were combined and washed with water (20 mL). twice, anhydrous MgSO 4 Drying, column chromatography (eluent: petroleum ether / ethyl acetate = 5 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com