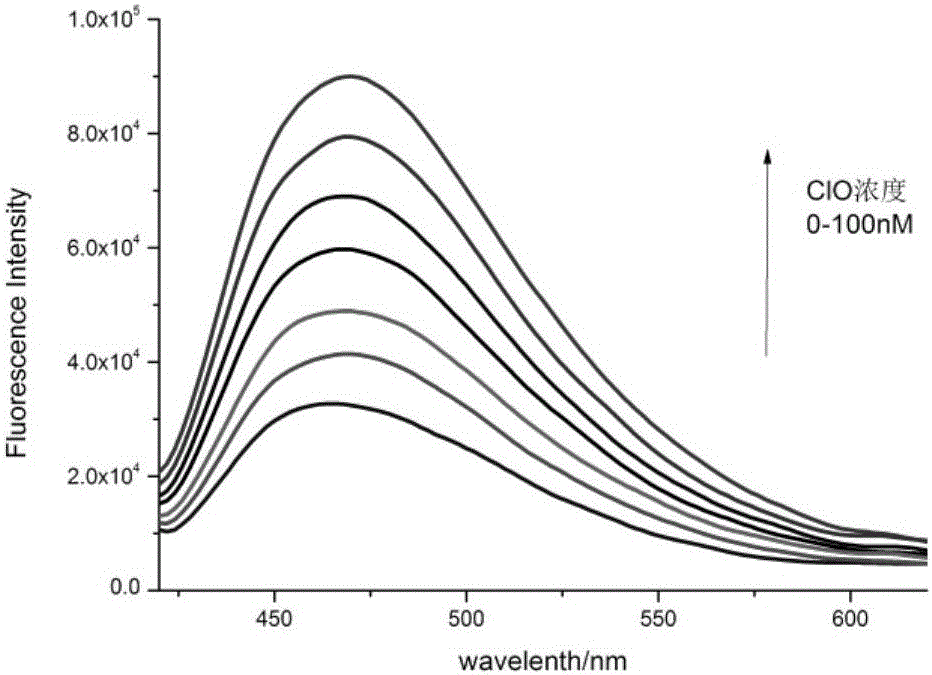
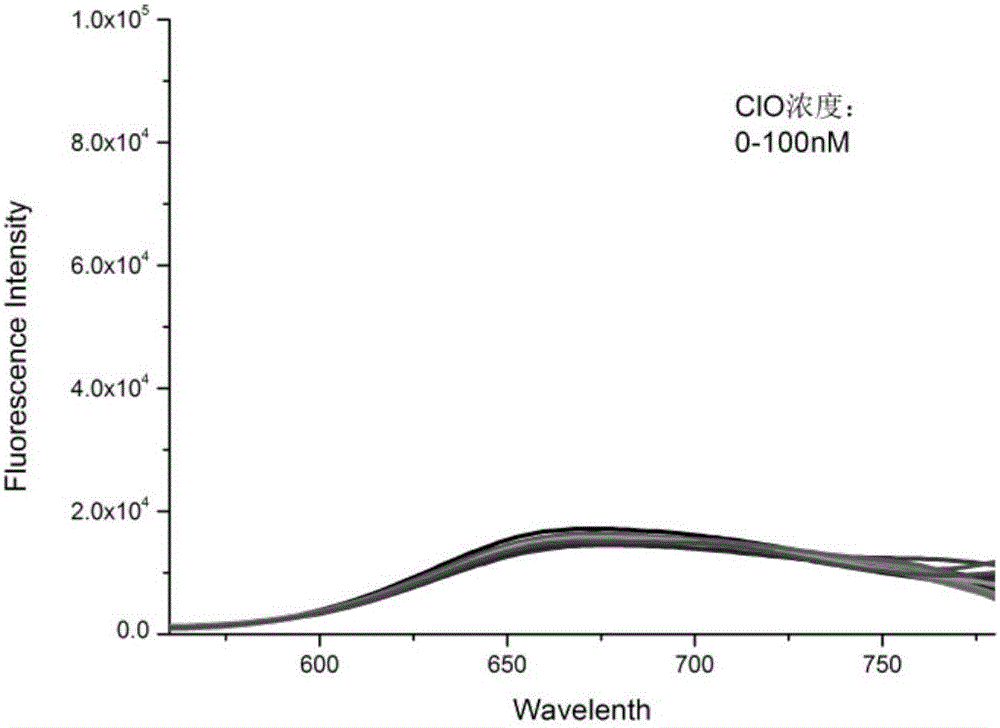
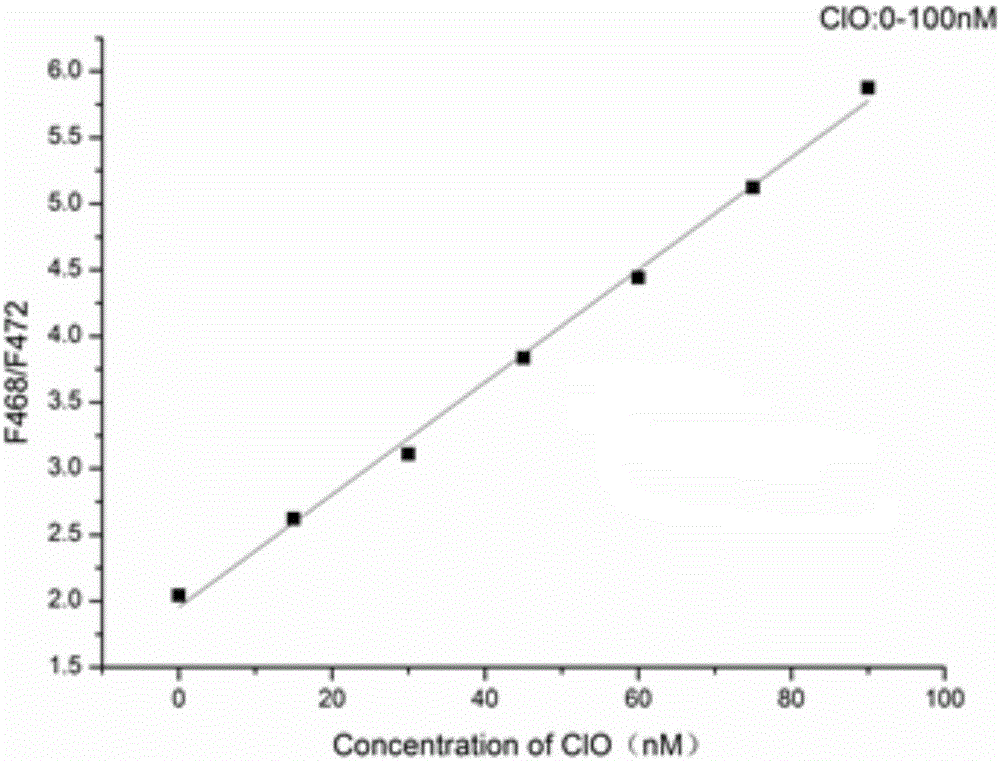
Fluorescent probe for detecting hypochlorous acid as well as preparation method and application thereof
A technology for detecting hypochlorous acid and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of inability to have both sensitivity and rapid response to two-photon, no two-photon and ratio properties, long reaction time, etc. Simple, easy-to-synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) 3-Hydroxy-9H-xanthen-9-one (182mg) and Lawson’s reagent (200mg) were dissolved in anhydrous toluene (4mL), refluxed at 75°C for 1.5h, filtered, spin-dried, and carried out on a chromatographic column Separation (eluent V 二氯甲烷 :V 甲醇 =25:1), spin-dried to obtain a reddish-brown solid (P).
[0044] NMR and mass spectrometry characterization:
[0045] 1 HNMR(400MHz,DMSO):δ=6.70(s,1H),6.89-6.91(d,J=8Hz,1H),7.43(s,1H),7.57-7.59(d,J=8Hz,1H),7.81 (s, 1H), 8.50-8.52 (d, J=8Hz, 1H), 8.64-8.65 (d, J=4Hz, 1H).
[0046] HRMS (ESI - ): m / z calcd for [M-H] = 227.0161,, Found 227.0153.
Embodiment 2
[0048](1) 3-Hydroxy-9H-xanthen-9-one (190mg) and Lawson’s reagent (200mg) were dissolved in anhydrous toluene (5mL), refluxed at 75°C for 1.5h, filtered, spin-dried, and carried out on a chromatographic column Separation (eluent V 二氯甲烷 :V 甲醇 =25:1), spin-dried to obtain a reddish-brown solid (P).
[0049] NMR and mass spectrometry characterization:
[0050] 1 HNMR(400MHz,DMSO):δ=6.70(s,1H),6.89-6.91(d,J=8Hz,1H),7.43(s,1H),7.57-7.59(d,J=8Hz,1H),7.81 (s, 1H), 8.50-8.52 (d, J=8Hz, 1H), 8.64-8.65 (d, J=4Hz, 1H).
[0051] HRMS (ESI - ): m / z calcd for [M-H] = 227.0161,, Found 227.0153.
Embodiment 3
[0053] This example is the same as Example 1, except that 3-hydroxy-9H-xanthen-9-one is changed to xanthenone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com