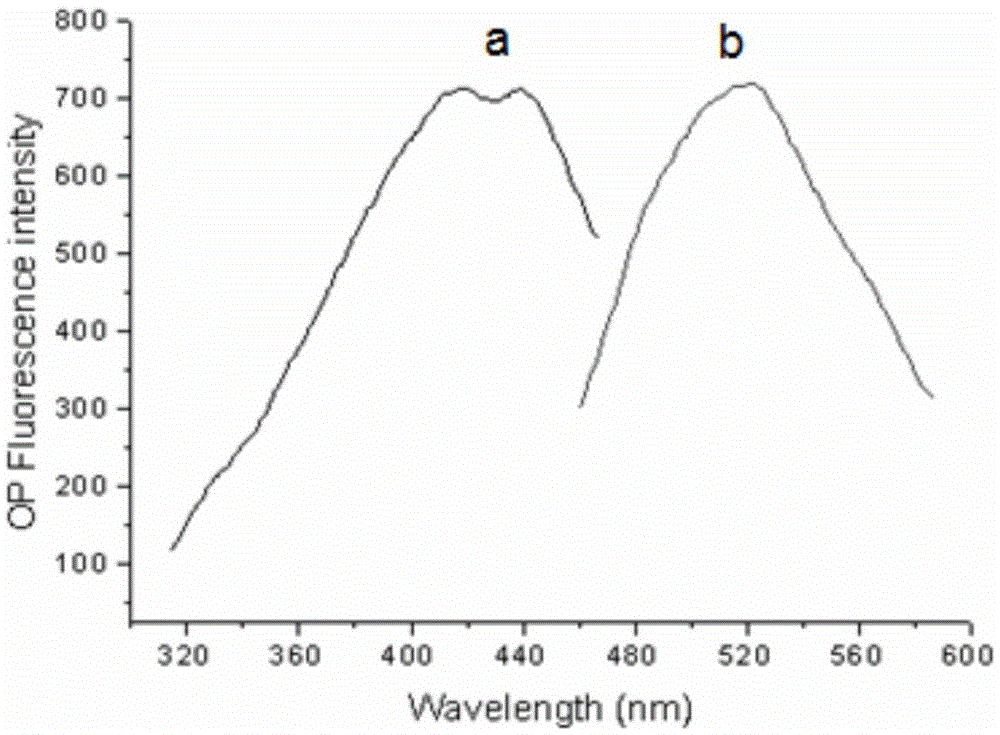
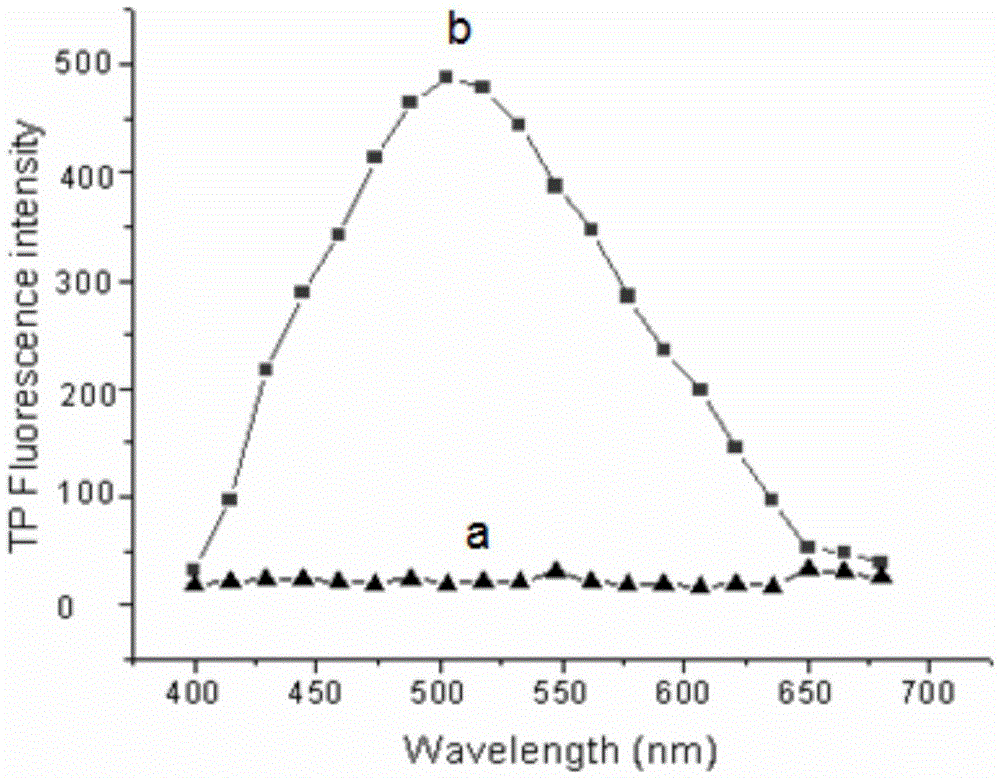
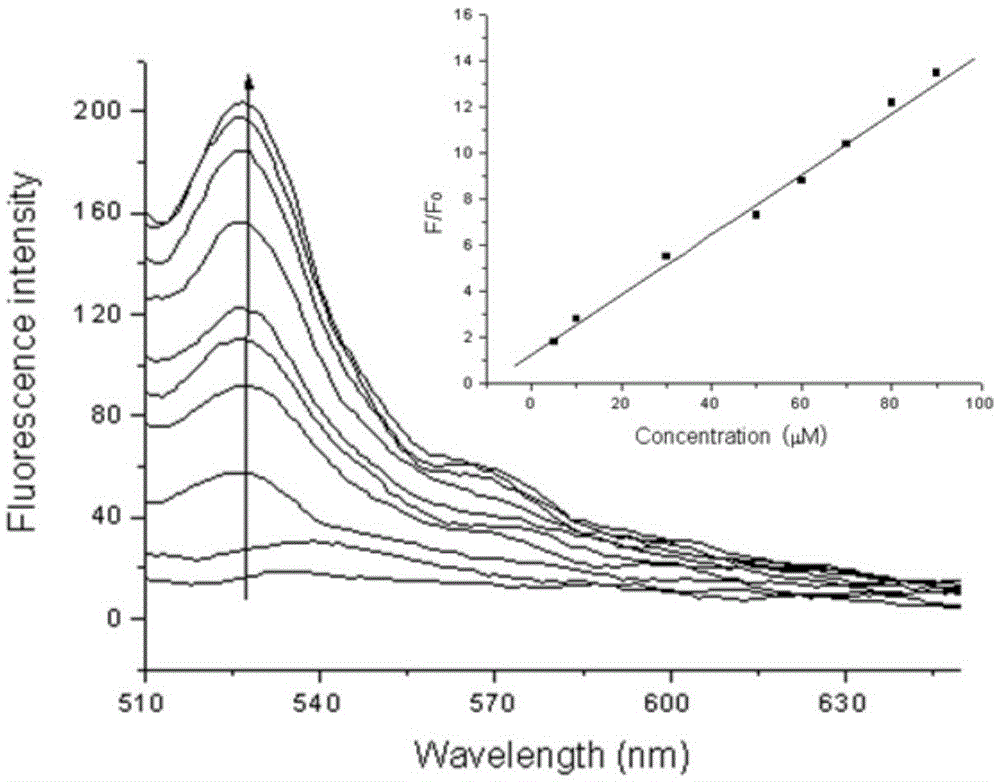
Two-photon reversible type fluorescent probe FO-PSe for hypochlorous acid detection, and preparation method and application thereof
A fluorescent probe and two-photon technology, applied in preparations for in vivo experiments, fluorescence/phosphorescence, chemical instruments and methods, etc., to achieve high yield, reduced self-absorption, and short dyeing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of fluorescent probe
[0038] Under the protection of argon, compound 1 (0.374g, 1.2mmol) and dithiothreitol (0.308g, 2.0mmol) were dissolved in 10-15mL of N,N-dimethylformamide and reacted at 75-100°C Add compound 2 (0.135g, 0.4mmol) after 30min, continue stirring for 20min, add 1,8-diazabicyclo[5.4.0]undec-7-ene (0.75ml, 5.0mmol), 75-100°C Reaction 25-40h. After the reaction was complete, the mixture was extracted with dichloromethane and saturated sodium bicarbonate, and the organic phase was dried over anhydrous magnesium sulfate. Then it was redissolved and subjected to column separation to obtain an orange-red solid (40%-80%).
[0039] NMR and mass spectrometry characterization:
[0040] 1 HNMR (400MHz, CDCl 3 ): δ7.594(s, 2H; ArH), 7.528(d, 2H; ArH), 7.456(d, 2H; ArH), 7.180-7.248(m, 10H; ArH). 13 CNMR (100MHz, CDCl 3 )δ191.54, 141.72, 137.18, 132.77, 132.53, 130.59, 128.60, 127.17, 127.06, 126.69, 119.94. 77SeNMR (150MHz, CDCl...
Embodiment 2
[0048] Embodiment 2: the synthesis of fluorescent probe
[0049] Under the protection of argon, compound 1 (0.374g, 1.2mmol) and dithiothreitol (0.308g, 2.0mmol) were dissolved in 1mL of N,N-dimethylformamide, reacted at 75°C for 30min, and then added the compound 2 (0.135g, 0.4mmol), continue stirring for 20min, add 1,8-diazabicyclo[5.4.0]undec-7-ene (0.75ml, 5.0mmol), and react at 75°C for 25h. After the reaction was complete, the mixture was extracted with dichloromethane and saturated sodium bicarbonate, and the organic phase was dried over anhydrous magnesium sulfate. Then it was re-dissolved and separated by column to obtain an orange-red solid (40%).
[0050] NMR and mass spectrometry characterization:
[0051] 1 HNMR (400MHz, CDCl 3 ): δ7.594(s, 2H; ArH), 7.528(d, 2H; ArH), 7.456(d, 2H; ArH), 7.180-7.248(m, 10H; ArH). 13 CNMR (100MHz, CDCl 3 )δ191.54, 141.72, 137.18, 132.77, 132.53, 130.59, 128.60, 127.17, 127.06, 126.69, 119.94. 77 SeNMR (150MHz, CDCl 3 ):δ431...
Embodiment 3
[0059] Embodiment 3: the synthesis of fluorescent probe
[0060] Under the protection of argon, compound 1 (0.374g, 1.2mmol) and dithiothreitol (0.308g, 2.0mmol) were dissolved in 15mL of N,N-dimethylformamide, reacted at 100°C for 30min, and then added the compound 2 (0.135g, 0.4mmol), continue stirring for 20min, add 1,8-diazabicyclo[5.4.0]undec-7-ene (0.75ml, 5.0mmol), react at 100°C for 40h. After the reaction was complete, the mixture was extracted with dichloromethane and saturated sodium bicarbonate, and the organic phase was dried over anhydrous magnesium sulfate. Then it was redissolved and separated by column to obtain an orange-red solid (80%).
[0061] NMR and mass spectrometry characterization:
[0062] 1 HNMR (400MHz, CDCl 3 ): δ7.594(s, 2H; ArH), 7.528(d, 2H; ArH), 7.456(d, 2H; ArH), 7.180-7.248(m, 10H; ArH). 13 CNMR (100MHz, CDCl 3 )δ191.54, 141.72, 137.18, 132.77, 132.53, 130.59, 128.60, 127.17, 127.06, 126.69, 119.94. 77 SeNMR (150MHz, CDCl 3 ):δ431.4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com