Method for displaying human arginase1 on surfaces of escherichia coli
An arginase and Escherichia coli technology, applied in the surface field of human arginase-1, can solve the problems of carrying various viruses, long application time, complicated preparation process, etc. The effect of reducing synthesis cost and simplifying purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
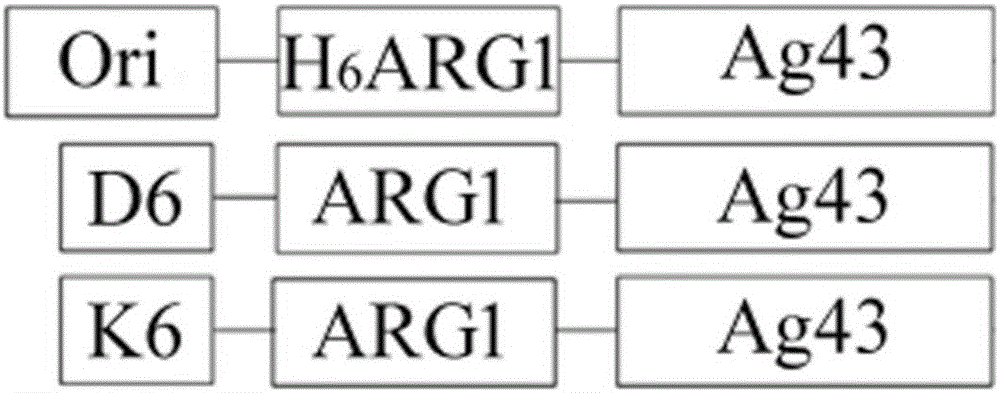
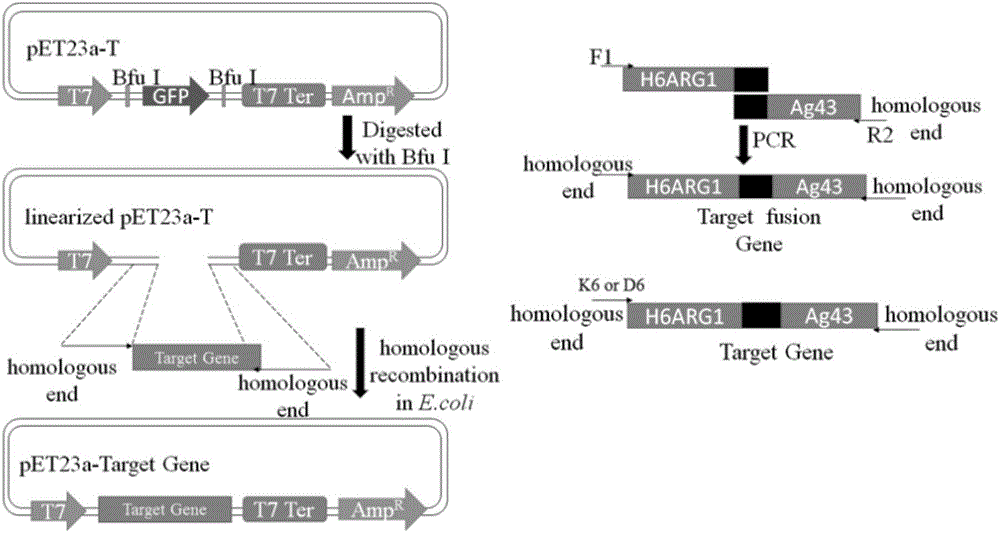
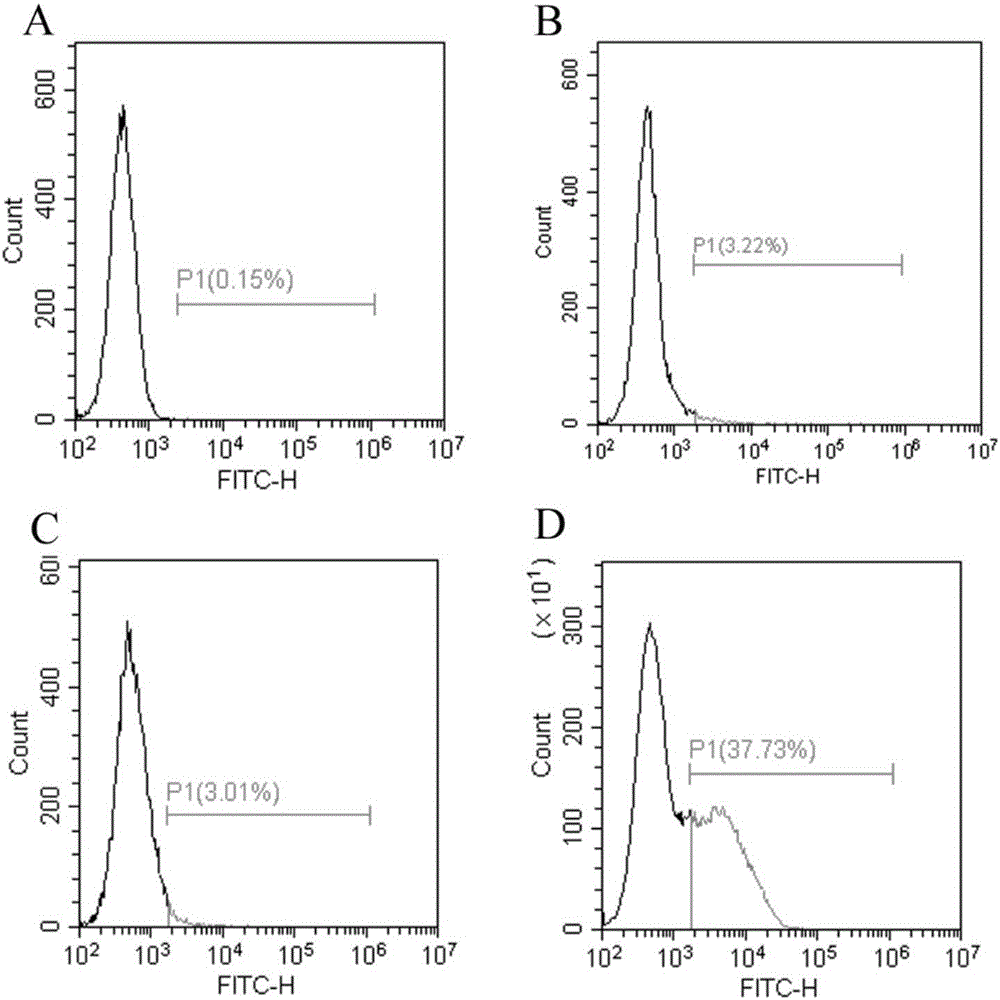
[0028] The method of the present invention is used to display human arginase-1 in Escherichia coli. First, the constructed recombinant plasmid pET23a / H 6ARG1-Ag43 transformed Escherichia coli competent cell Rosetta Blue strain, and cultured overnight at 37°C. Then, pick a single colony and inoculate it in 100mL LB medium. The final concentration of antibiotic ampicillin is 100μg / mL. Shake culture at 37°C. The OD value is between 0.5 and 0.6. Incubate for 8 hours. Then, the cells were collected by centrifugation at 12000 RPM at 4°C, washed with pre-cooled PBS for 3 times, and finally resuspended in 10 mL of pre-cooled PBS buffer for later use. Then it was measured by Chinard reaction and converted to the corresponding human arginase-1 enzyme activity. ( picture 3C , picture 4 )
Embodiment 2
[0030] The method of the present invention is used to display human arginase-1 in Escherichia coli. First, the constructed recombinant plasmid pET23a / H 6 D. 6 - ARG1-Ag43 was transformed into Escherichia coli competent cell Rosetta Blue strain, and cultured overnight at 37°C. Then, pick a single colony and inoculate it in 100mL LB medium, the final concentration of antibiotic ampicillin is 100μg / mL, shake culture at 37°C, the OD value is between 0.5 and 0.6, add IPTG, the final concentration is 1mM, 37°C Induction culture was carried out for 8 hours. Then, the cells were collected by centrifugation at 12000 RPM at 4°C, washed with pre-cooled PBS for 3 times, and finally resuspended in 10 mL of pre-cooled PBS buffer for later use. Then it was measured by Chinard reaction and converted to the corresponding human arginase-1 enzyme activity. ( picture 3B , picture 4 )
Embodiment 3
[0032] The method of the present invention is used to display human arginase-1 in Escherichia coli. First, the constructed recombinant plasmid pET23a / H 6 K 6 - ARG1-Ag43 was transformed into Escherichia coli competent cell Rosetta Blue strain, and cultured overnight at 37°C. Then, pick a single colony and inoculate it in 100mL LB medium. The final concentration of antibiotic ampicillin is 100μg / mL. Shake culture at 37°C. The OD value is between 0.5 and 0.6. Incubate for 8 hours. Then, the cells were collected by centrifugation at 12000 RPM at 4°C, washed with pre-cooled PBS for 3 times, and finally resuspended in 10 mL of pre-cooled PBS buffer for later use. Then it was measured by Chinard reaction and converted to the corresponding human arginase-1 enzyme activity. ( picture 3D , picture 4 )
[0033] Various example result analysis
[0034] After flow cytometry analysis, the charged polypeptide 6×Glu and 6×Lys can improve the display efficiency of Antigen 43 protein...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com