Method and kit for detecting HBV PgRNA in blood of patient with hepatitis b and application thereof
A hepatitis B, kit technology, applied in the field of molecular biology, can solve problems such as unrealizable, and achieve the effects of convenient use, reliable detection results, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] 1. Sample collection: collect serum or plasma.
[0088] 1) Serum sample collection: Use a sterile syringe to draw 3-5 mL of venous blood from the bend of the subject’s arm or the back of the hand, inject it into a sterile collection tube, and leave it at room temperature for no more than 4 hours until the sample precipitates serum by itself or directly centrifuge at 1600 rpm at room temperature After 5 minutes, the serum was separated and transferred to a 1.5mL sterilized centrifuge tube for later use.
[0089] 2) Plasma sample collection: Use a sterile syringe to draw 3-5 mL of venous blood from the bend of the subject's arm or the back of the hand, inject it into a sterile collection tube containing EDTA, immediately invert and mix it evenly, and place it at room temperature for no more than 4 hours. Wait for the sample to precipitate the plasma by itself or directly centrifuge at room temperature at 1600rpm for 5 minutes to separate the plasma, and transfer it to a 1...
Embodiment 2
[0132] Design of primers and probes:
[0133] At present, the HBV genotypes in my country are mainly B and C, with a small amount of D, and a small amount of fusion types of the above genotypes.
[0134] In the present invention, when designing primers and probes, B-type, C-type and D-type are taken into account. Referring to the sequences of all HBV types B, C and D in the internationally recognized database NCBI, after comparison, the sequence part of PgRNA that is different from other mRNAs, that is, the part between about 1800bp and 2800bp, was selected for analysis. The designed primers and probes are located in the conserved or relatively conserved region sequences of each genotype of HBV, ensuring the compatibility of the "primers and probes used in the patent" to various common genotypes of HBV in China. Primers with base sequences shown in SEQ ID NO: 1-NO: 6 and probes with base sequences shown in SEQ ID NO: 7 were designed.
[0135] Screening of primers and probes:...
Embodiment 3
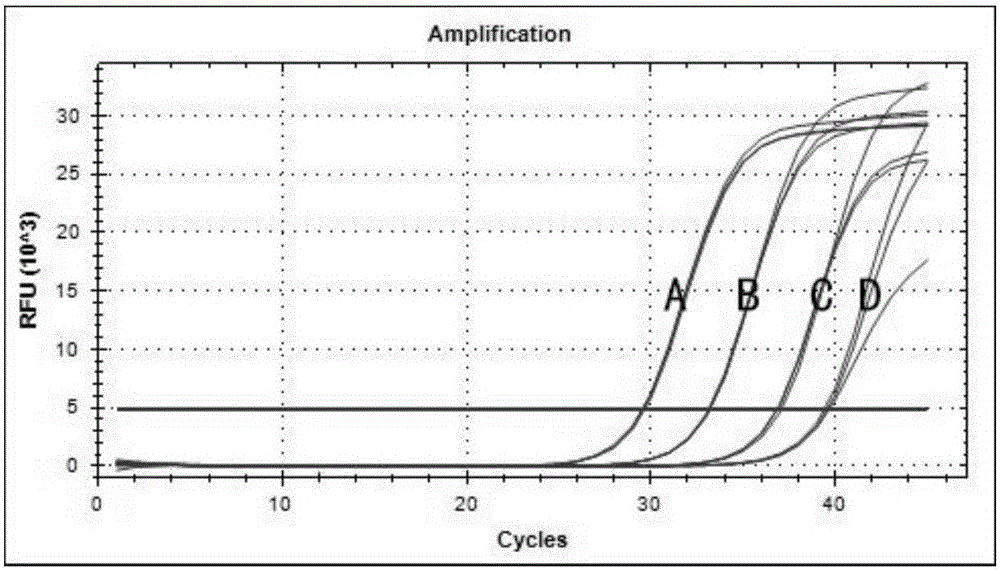
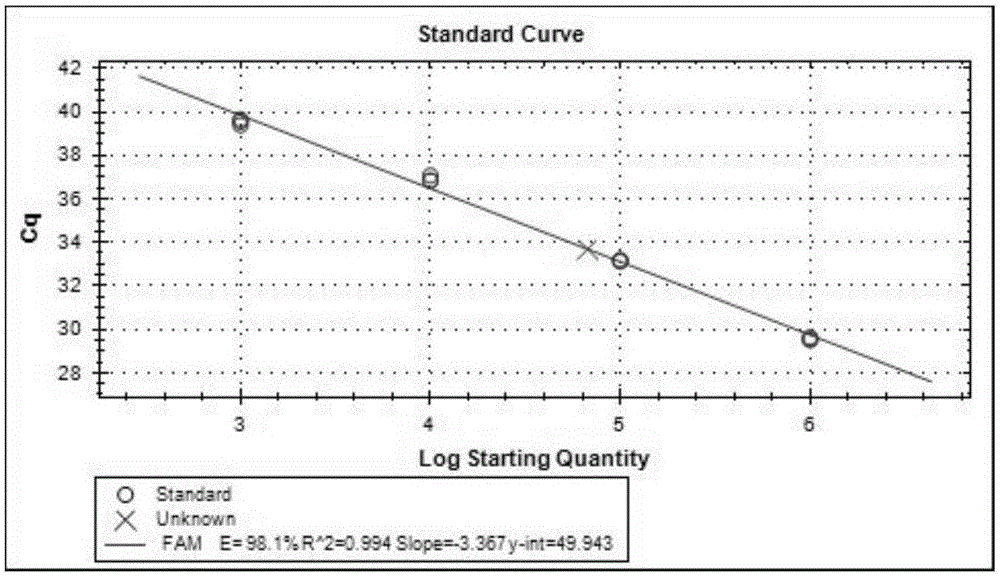
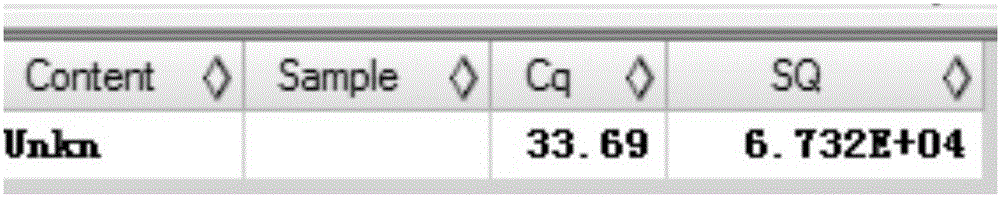
[0146] DNA Amplification Verification of HBV pgRNA Extract
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com