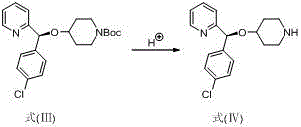
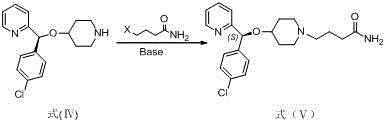
Preparation method of anti-allergic drug bepotastine
A bepotastine and anti-allergic technology, which is applied in the field of synthesis of anti-allergic drugs, can solve the problems of cumbersome operation, high cost, and difficulty in large-scale preparation, and achieve the effect of low cost and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
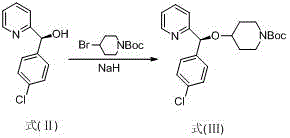
[0025] Preparation of (S)-N-tert-butoxycarbonyl-4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidine (formula III)
[0026]
[0027] Dissolve 21.9 g (0.1 mol) of compound (S)-(4-chlorophenyl)(2-pyridyl)-methanol formula (Formula II) in 160 mL of tetrahydrofuran, and add 60% mass fraction of hydrogenation solution in batches under stirring Sodium 41g (0.1mol), stirred for 2 hours until no bubbles emerged, and the reaction liquid was added dropwise to the solution of N-tert-butoxycarbonyl-4-bromopiperidine 26.4g (0.1mol) under ice bath cooling, and added dropwise After completion, the temperature was gradually raised to room temperature under stirring for 5 hours, the reaction solution was added to 200mL saturated ammonium chloride solution, stirred for 30 minutes, the reaction solution was extracted twice with 300mL ethyl acetate, the organic phases were combined, and dried over anhydrous sodium sulfate for 2 hours , filtered, and the mother liquor was rotary evaporated to dryne...
Embodiment 2
[0029] Preparation of (S)-N-tert-butoxycarbonyl-4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidine (formula III)
[0030] The sodium hydride in embodiment 1 is replaced with the sodium ethylate of equimolar amount, other operations are the same as embodiment 1, and product yield is 73%.
Embodiment 3
[0032] Preparation of (S)-N-tert-butoxycarbonyl-4-[(4-chlorophenyl)(2-pyridyl)methoxy]piperidine (formula III)
[0033] The tetrahydrofuran in Example 1 was replaced with an equivalent amount of N,N-dimethylformamide, and other operations were the same as in Example 1, and the product yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com