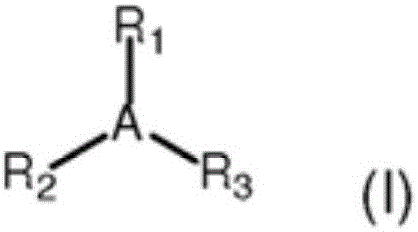
Process for the production of modified butyl rubber
A technology of derivatives and ionomers, applied in the field of modified ionomers and their production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
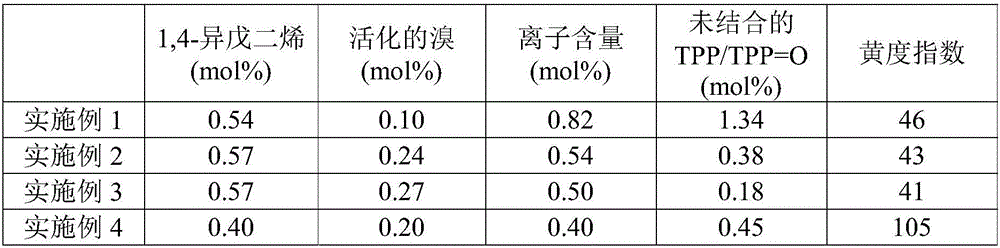
[0053] The ionomers produced according to the method of the present invention have improved color properties which make the ionomers particularly suitable for use in films and coatings. The yellowness index, as defined in ASTM E313, is a measure of how far an object deviates toward yellow from the preferred white. According to one embodiment of the present invention, as measured according to ASTM E313, the yellowness index of the polymer is between about 1-100, preferably between about 10-70, more preferably between about 20-60, even more Preferably between about 20-41.
[0054] Without being bound by a particular theory, it is believed that the yellowness index may be at least partially related to polymer degradation, as indicated by the level of isoprene in the final polymer. Degradation of ionomers is most facile at the 1,4-isoprene polyene segment, as opposed to the isobutylene segment of the polymer chain. A decrease in the level of 1,4-isoprene thus indicates degradati...
Embodiment 1
[0079] Example 1: Comparative example, as described in US Patent 7,662,480:
[0080] Add 48g (100phr) of LANXESS BB2030 TM and 4.7 g (9.7 phr, 3 molar equivalents, based on the allyl bromide content) of triphenylphosphine were added to a Brabender internal mixer (capacity 75 g) operated at 100°C and a rotor speed of 60 rpm. Mixing was carried out for a total of 60 minutes. The properties obtained are shown in Table 2, most notably, significant amounts of residual / unbound TPP and its oxidized derivative triphenylphosphine oxide (TPP=O).
Embodiment 2
[0081] Example 2: Comparative example, as described in WO 2012083419:
[0082] Allow mixing LANXESS BB2030 alone before adding TPP (4.3phr) TM (100phr) for a short time, then mix for 10 minutes. The properties obtained are shown in Table 2, most notably the residual / unbound TPP and its oxidized derivative triphenylphosphine oxide (TPP=O) showing 65% conversion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com