Method for detecting content of free sodium hyaluronate in medical cross-linking sodium hyaluronate gel
A technology of cross-linking hyaluronic acid and sodium hyaluronate, which is applied in the field of chemical testing and pharmaceutical analysis to achieve good reproducibility, small error and accurate experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Experimental method and process:
[0018] (1) Sample preparation steps
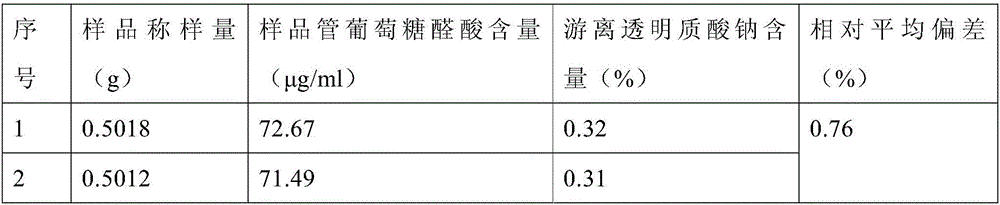
[0019] Take 0.5 g of cross-linked sodium hyaluronate gel in a vial, add 5 mL of PBS to shake evenly, filter the solution through a filter membrane (pore size 5 μm), rinse the vial with 5 mL of PBS, take 1 mL of the filtrate, and measure the content of glucuronic acid. Determination.
[0020] (2) Preparation steps of glucuronic acid standard solution series
[0021] Glucuronic acid (GA) standard solution: Accurately weigh 10mg of glucuronic acid in a 100mL volumetric flask, dilute to the mark with water, shake well, and store at 4°C. Prepare the glucuronic acid standard series as shown in Table 1.
[0022] Table 1
[0023] Test tube number
0
1
2
3
4
5
GA standard solution, mL
0
0.2
0.4
0.6
0.8
1.0
Distilled water, mL
1.0
0.8
0.6
0.4
0.2
0
GA content, μg / mL
0
20
40
60
80
100
[0024] (3) De...
Embodiment 2
[0031] Experimental method and process:
[0032] (1) Sample preparation steps
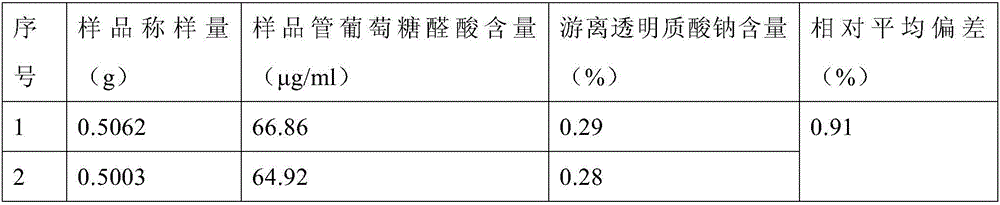
[0033] Take 0.5 g of cross-linked sodium hyaluronate gel in a vial, add 5 mL of PBS to shake evenly, filter the solution through a filter membrane (pore size 3 μm), rinse the vial with 5 mL of PBS, take 1 mL of the filtrate, and measure the content of glucuronic acid. Determination.
[0034] (2) Preparation steps of glucuronic acid standard solution series
[0035] Glucuronic acid (GA) standard solution: Accurately weigh 10mg of glucuronic acid in a 100mL volumetric flask, dilute to the mark with water, shake well, and store at 4°C. Prepare the glucuronic acid standard series as shown in Table 3 below.
[0036] table 3
[0037] Test tube number
0
1
2
3
4
5
GA standard solution, mL
0
0.2
0.4
0.6
0.8
1.0
Distilled water, mL
1.0
0.8
0.6
0.4
0.2
0
GA content, μg / mL
0
20
40
60
80
100
[0038] ...
Embodiment 3
[0045] Experimental method and process:
[0046] (1) Sample preparation steps
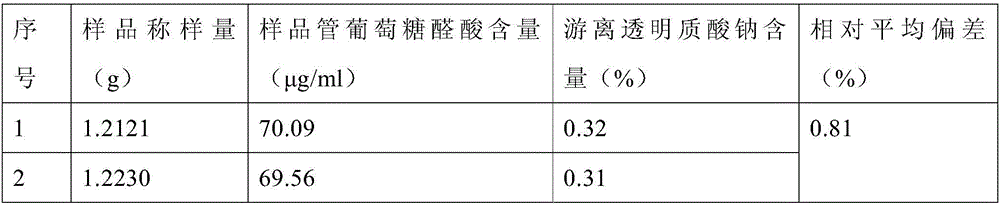
[0047] Take 1.25 g of cross-linked sodium hyaluronate gel in a vial, add 15 mL of PBS to shake evenly, filter the solution through a filter membrane (pore size 5 μm), rinse the vial with 10 mL of PBS, take 1 mL of the filtrate, and measure the content of glucuronic acid. Determination.
[0048] (2) Preparation steps of glucuronic acid standard solution series
[0049] Glucuronic acid (GA) standard solution: Accurately weigh 10mg of glucuronic acid in a 100mL volumetric flask, dilute to the mark with water, shake well, and store at 4°C. Prepare the glucuronic acid standard series as shown in Table 5.
[0050] table 5
[0051] Test tube number
0
1
2
3
4
5
GA standard solution, mL
0
0.2
0.4
0.6
0.8
1.0
Distilled water, mL
1.0
0.8
0.6
0.4
0.2
0
GA content, μg / mL
0
20
40
60
80
100
[0052] (3)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com