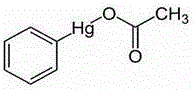
Applications of phenylmercuric acetate in serving as indoleamine 2,3-dioxygenase-1 inhibitor
A technology of phenylmercuric acetate and dioxygenase, which is applied in the application field of diseases, can solve the problems that laboratory or clinical effects need to be improved, the number of IDO1 inhibitors is small, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
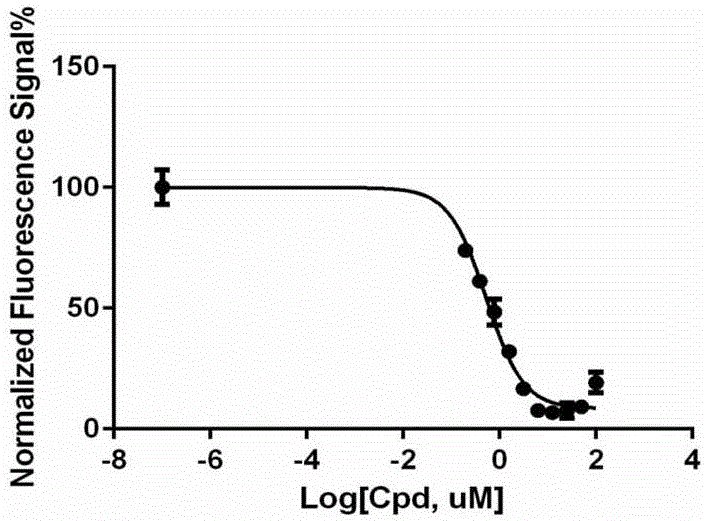
[0015] The present invention will now be further explained in conjunction with the following experiments and accompanying drawings. It should be noted that these experimental examples and accompanying drawings should not be construed as limiting the present invention.
[0016] Kynurenine standard curve drawing
[0017] 1. Standard medium (200 μL): 20 μL of 0.5M potassium phosphate buffer (pH 6.5), with a final concentration of 50 mM; 20 μL of 0.2M ascorbic acid, with a final concentration of 20 mM; 4 μL of 0.5 mM methylene blue, with a final concentration of 10 μM; 4 μL of 5 mg / ml catalase to a final concentration of 100 μg / ml; 132 μL of dd-H 2 O; 20 μL of kynurenine solution with final concentrations of 0, 1, 5, 7.5, 10, 25, 50, 75 and 100 mM.
[0018] 2. After adding 40μL of 1M NaOH solution, centrifuge the medium (11,500rpm, 4°C, 15min).
[0019] 3. Transfer 200 μL of the supernatant to a 96-well microtiter plate and measure the fluorescence intensity (λex 360nm, λem480nm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com