Bridged bispecific fusion protein
A fusion protein and bispecific technology, applied in the direction of hybrid immunoglobulin, fusion polypeptide, immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1 Construction of recombinant expression plasmids for dual-target fusion proteins
[0097] 1. Preparation of DNA fragments of VEGFR and FGFR
[0098] VEGF receptor and FGF receptor cDNA fragments were obtained by synthesis (synthesized by Nanjing GenScript Biotechnology Co., Ltd.), and the sequences are shown in SEQ ID NO: 14 and SEQ IN NO: 16. The synthesized cDNA fragment was directly synthesized onto the PUC57-vector (the vector can be purchased from Nanjing GenScript Biotechnology Co., Ltd.).
[0099] 2. Preparation of DNA Fragment of Long Adapter Fc
[0100] Fc-cDNA sequence was synthesized, and the synthesized Fc had adapters (synthesized by Nanjing GenScript Biotechnology Co., Ltd.) before and after, which was synthesized on the vector HX1 (the vector can be purchased from Nanjing GenScript Biotechnology Co., Ltd.) , thereby obtaining an Fc DNA fragment with a long linker, also referred to as Fc long herein. Specifically, the front and back joints are:...
Embodiment 2
[0121] Example 2 Transient expression of fusion protein
[0122]Use the MAX plasmid purification kit (QIAGEN) to purify the corresponding fusion protein plasmid DNA, determine the concentration of the plasmid DNA with an ultraviolet spectrophotometer, and culture 1 μg of the recombinant plasmid and 6 μL of liposomes (FuGENE 6 Transfection Reagent, Roche Company) in 100 μL of fresh IMDM solution (GIBCO company), mix well, let it stand for 15 minutes, then add 3×10 cells according to cell density 5 / mL inoculated in CHO cells (ATCC) cultured overnight in a 6-well plate, the complete culture medium of the cells contained 88% IMDM, 10% FBS, 1% HT and 1% glutamine (both products of GIBCO Company), at 37 °C, 5% CO 2 After culturing in the incubator for 48 hours, the supernatant was collected, and the relative content of the fusion protein secreted and expressed by CHO was determined with a human IgG ELISA protein quantification kit (BETHYL Company).
Embodiment 3
[0123] Example 3 Determination of binding affinity of fusion protein
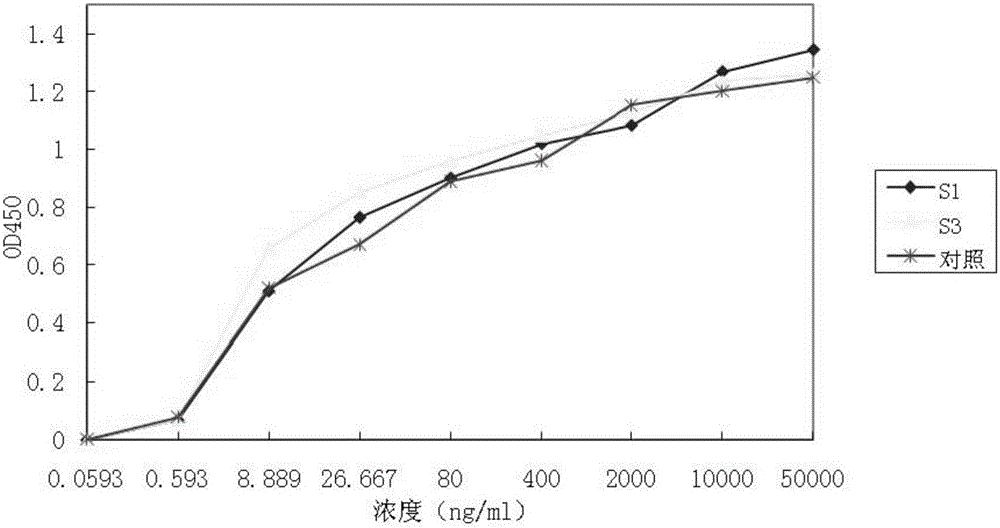
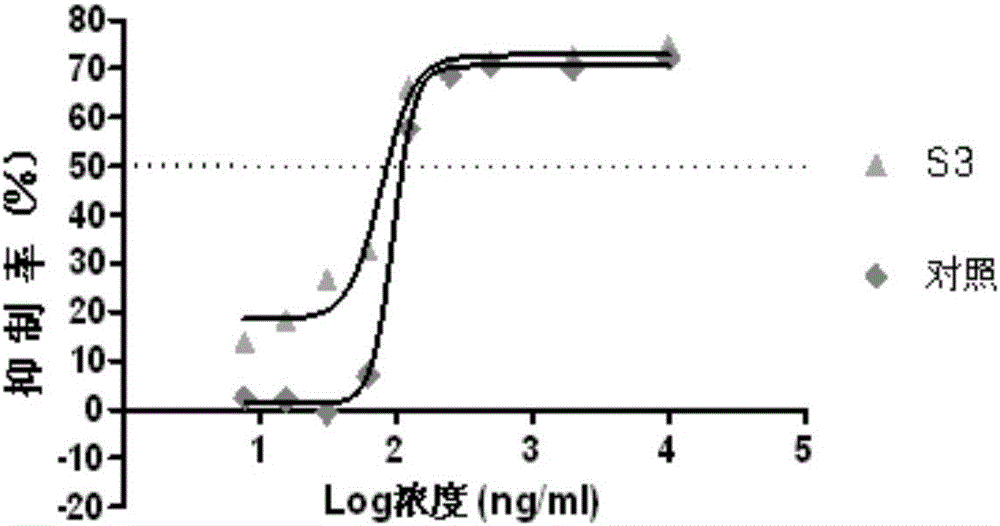
[0124] The binding ability of the fusion protein constructed above to VEGF and FGF-2 was detected by ELISA method. Coat the plate with 20ng / well VEGF or 50ng / well FGF-2, 100μl / well, overnight at 2-8°C. Wash the plate 3 times in a plate washer. 3% BSA-PBST solution blocked, 37 degrees 2h. Wash the plate 3 times in a plate washer. Loading: use PBST solution to dilute the marked line from 10000ng / ml (for VEGF-coated plates) or 50000ng / ml (for FGF-coated plates) to 9 points of serial dilution, 100μl / well, 37 degrees for 1h. Wash the plate 3 times in a plate washer. Dilute the secondary antibody (Goat anti-human IgG-Fc-HRP) 5000 times with PBST solution. Add TMB color developing solution to develop color, and keep away from light for 5 min at room temperature. with 2M H 2 SO 4 The test was terminated, and the optical density absorbance value was read with a 450nm microplate reader. Wherein, the VEGFR-FG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com