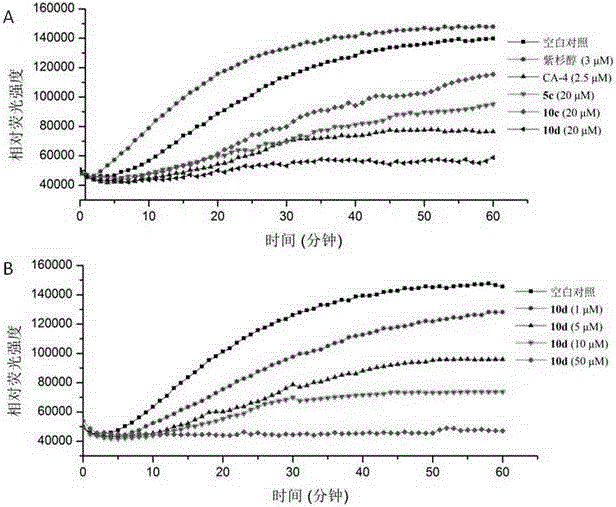
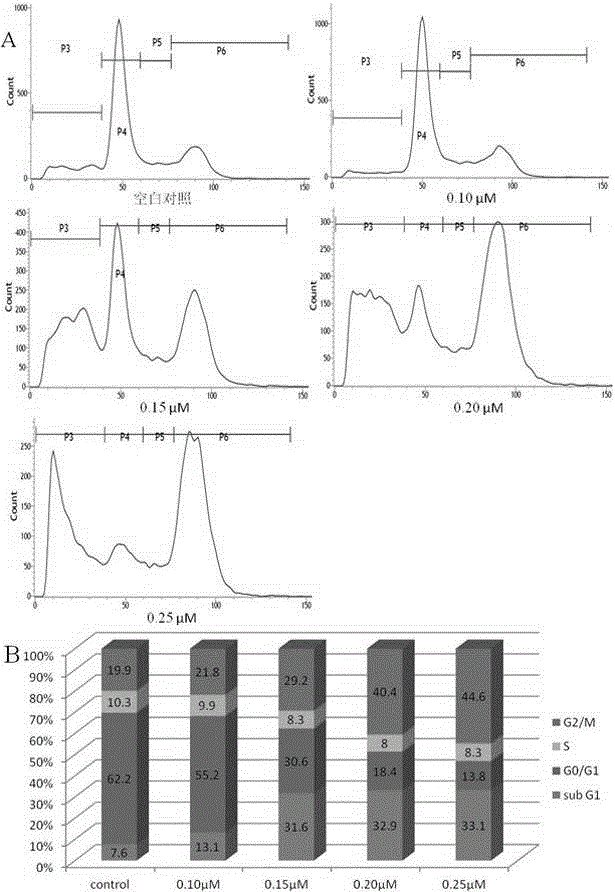
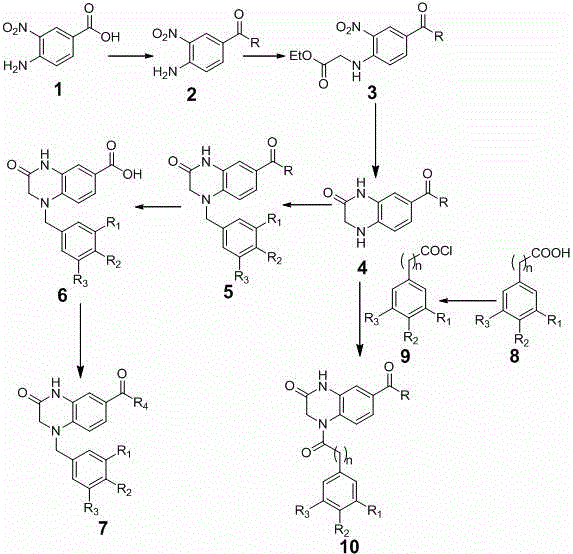
Nitrogen substitutive 3-oxygen substitutive-6-tetrahydroquinoxaline substitutive structure compound and preparation method and medical application thereof
A kind of technology of tetrahydroquinoxaline and compound, applied in the field of medicine, can solve problems such as poor water solubility, limited clinical application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of 1-(4-methoxybenzyl)-3-oxo-1,2,3,4-tetrahydroquinoxaline-6-carboxylic acid methyl ester (5a)
[0048]
[0049] (1) Preparation of 3-nitro-4-aminobenzoic acid methyl ester (2a)
[0050] Put 3-nitro-4-aminobenzoic acid (that is, compound 1, 4.50g, 24.7mmol) and anhydrous methanol (80mL) into a 250mL three-necked bottle in turn, and when the temperature dropped to 0°C, drop Slowly add thionyl chloride (5.38mL, 74.1mmol, 3equiv) dropwise into the liquid funnel, after the dropwise addition, continue to stir at 0°C for 30 minutes, then raise the temperature to 50°C for 4h, a large amount of yellow solid is produced, suction filter, and the filtrate The methanol in the mixture was evaporated to dryness, 30 mL of water and 30 mL of ethyl acetate were added, the layers were separated, the organic layer was dried with anhydrous sodium sulfate, and the solvent was evaporated to dryness to obtain a yellow solid, which was combined with the previous filter cake and ...
Embodiment 2
[0058] Preparation of 1-(3,4-dimethoxybenzyl)-3-oxo-1,2,3,4-tetrahydroquinoxaline-6-carboxylic acid methyl ester (5b)
[0059]
[0060] Except that 3,4-dimethoxybenzyl chloride was used instead of 4-methoxybenzyl chloride in step (4), other steps and purification methods were the same as in Example 1 to obtain 29 mg of white solid with a yield of 56.1%. Melting point: 155.9-157.5°C. 1 H NMR (300MHz, CDCl 3 )δ9.05(s,1H),7.65(dd,J=8.5,1.8Hz,1H),7.50(d,J=1.8Hz,1H),6.82(s,2H),6.79-6.74(m,2H ),4.43(s,2H),3.92(s,2H),3.87(s,6H),3.83(s,3H). 13 C NMR (75MHz, CDCl 3 )δ166.8, 165.8, 149.5, 148.7, 138.9, 127.6, 126.6, 125.2, 120.0, 119.9, 116.8, 111.4, 110.9, 110.5, 56.0, 53.2, 52.0, 51.6. HRMS (ESI): calcd for C 19 h 21 N 2 o 5 [M+H] + 357.14450, found 357.14450.
Embodiment 3
[0062] Preparation of 1-(2,3,4-trimethoxybenzyl)-3-oxo-1,2,3,4-tetrahydroquinoxaline-6-carboxylic acid methyl ester (5c)
[0063]
[0064] Except that 3,4,5-trimethoxybenzyl chloride was used instead of 4-methoxybenzyl chloride in step (4), other steps and purification methods were the same as in Example 1 to obtain 38 mg of white solid with a yield of 67.8% . Melting point: 188.9-191.0°C. 1 H NMR (300MHz, CDCl 3 )δ8.02(brs,1H),7.67(dd,J=8.5,1.9Hz,1H),7.43(d,J=1.9Hz,1H),6.78(d,J=8.6Hz,1H),6.47( s,2H),4.42(s,2H),3.93(s,2H),3.88(s,3H),3.84(s,3H),3.81(s,6H). 13 C NMR (75MHz, CDCl 3 )δ166.7, 165.7, 153.8, 138.8, 137.5, 131.0, 126.7, 125.1, 120.2, 116.8, 111.0, 104.3, 60.9, 56.2, 53.8, 52.0, 51.8. HRMS (ESI): calcd for C 20 h 23 N 2 o 6 [M+H] + 387.15506,found 387.15512.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com