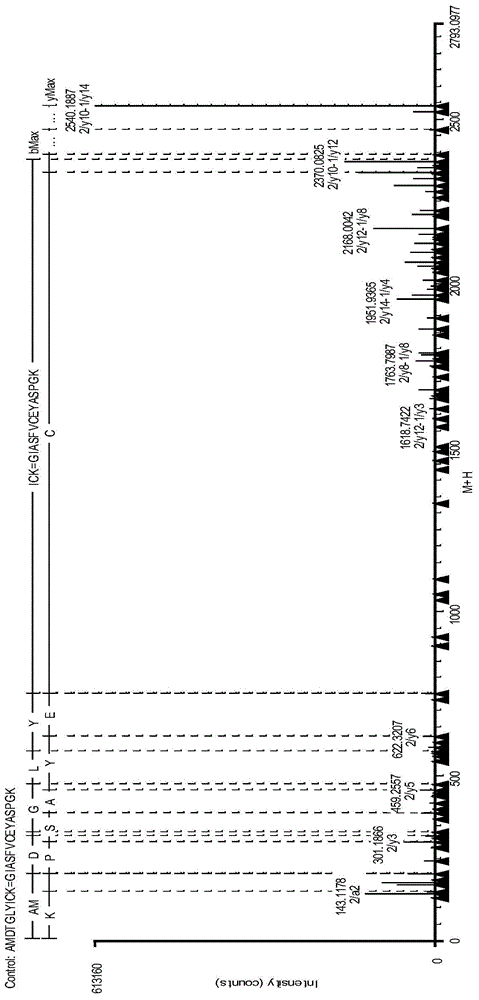
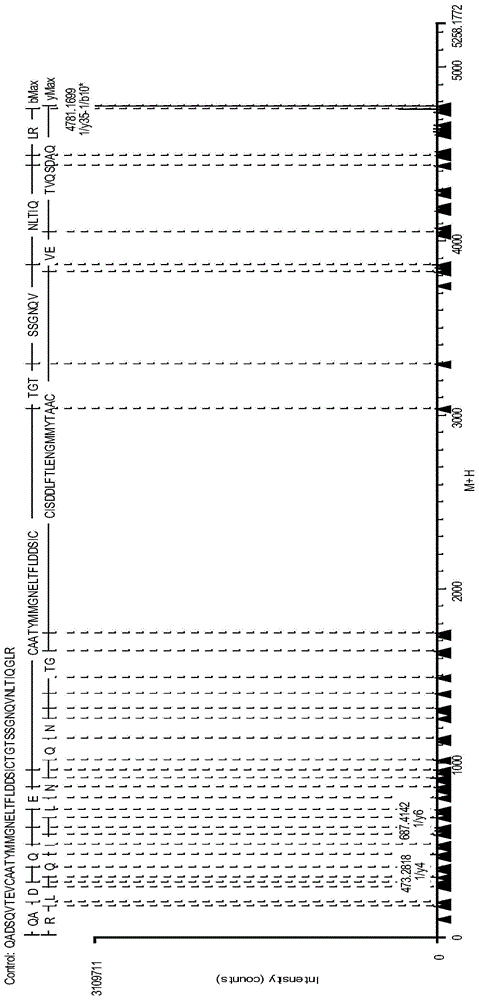
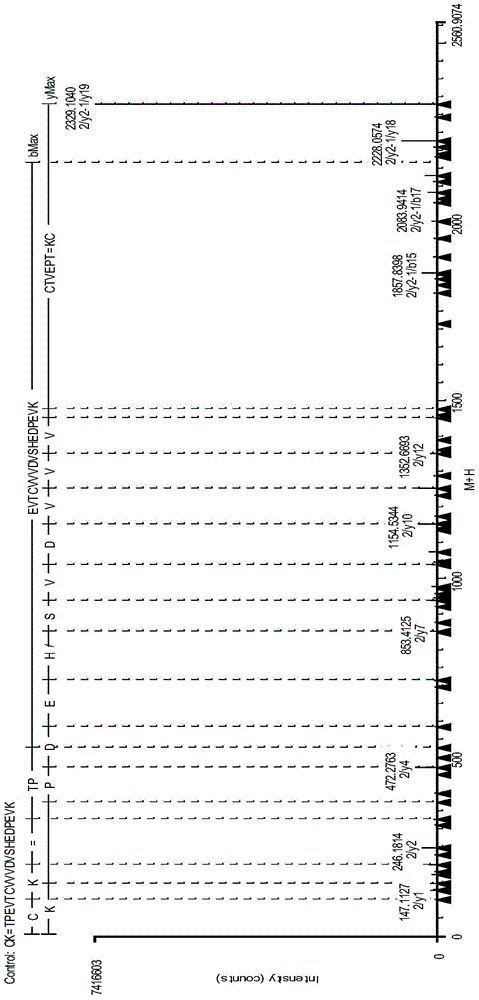
Analysis method for disulfide bond pairing of fusion protein
A fusion protein and analysis method technology, applied in the direction of analysis materials, material separation, measurement devices, etc., can solve the problems of many types of enzyme digestion, inconvenient, and insufficient fragment ions, etc., to achieve rich fragment ions and few enzyme digestion methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Fusion protein disulfide bond pairing analysis (trypsin digestion)
[0046] Fusion protein disulfide bond pairing analysis steps:
[0047] (1) Removal of N-Glycosylations
[0048] Take 1mg rhCTLA4-Ig fusion protein sample, add 50mM NH 4 HCO 3 (pH 8.0) solution to a volume of 20-50 μL, add 1 μL of peptide N-glycosidase PNGase F (NEB, 500000u / mL), mix well, and incubate at 37°C for 24 hours.
[0049] (2) Protein denaturation treatment
[0050] Take 0.5 mg of the protein sample incubated in (1) and add it to 380 μl of denaturation buffer (8M guanidine hydrochloride+5mM EDTA+0.5M Tris, pH8.3), mix well, and incubate at 37°C for 60 minutes. Ultrafiltration centrifugation or desalting column desalting to 50mM NH 4 HCO 3 (pH8.0) buffer.
[0051] (3) Enzymolysis
[0052] Take 100 μg of the protein in (2), add trypsin to digest the antibody protein at 1:25 (wt / wt), and incubate at 37°C for 4 hours.
[0053] (4) Reduction and termination of enzymatic hydrolysi...
Embodiment 2
[0068] Example 2: Analysis of the complete molecular weight of the fusion protein (trypsin combined with chymotrypsin digestion)
[0069] Fusion protein disulfide bond pairing analysis steps:
[0070] (1) Removal of N-Glycosylations
[0071] Take 1mg rhCTLA4-Ig fusion protein sample, add 50mM NH 4 HCO 3 (pH8.0) solution to a volume of 20-50 μL, add 1 μL of peptide N-glycosidase PNGase F (NEB, 500000u / mL), mix well, and incubate at 37°C for 24 hours.
[0072] (2) Protein denaturation treatment
[0073] Take 0.5mg of the protein sample incubated in (1) and add it to 380ul denaturation buffer (8M guanidine hydrochloride+5mM EDTA+0.5M Tris, pH8.3), mix well, and incubate at 37°C for 60 minutes. Ultrafiltration centrifugation or desalting column desalting to 50mM NH 4 HCO 3 (pH8.0) buffer.
[0074] (3) Enzymolysis
[0075] Take 100 μg of the protein in (2), add trypsin at 1:25 (wt / wt), add chymotrypsin at 1:50 (wt / wt) to digest the protein, and incubate at 37°C for 4 hours....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com