A kind of azepine-2,7-dione acceptor unit and its application
A receptor unit, azepine technology, applied in the field of azepine-2,7-dione receptor unit, which can solve the problems of exhaustion of fossil fuels and the impact of fossil fuels on global climate and environment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: attributed to the preparation of the acceptor unit TTA-Br of general formula (I) structure
[0086]
[0087] The starting material thieno[3,2-b]thiophene-3-carboxylate ethyl ester (1), the preparation of TMPMgCl LiCl can refer to the literature synthesis (Selective Multiple Magnesiations of the Thieno[3,2-b]thiophene Scaffold.P. Knochel et al, Chem. Eur. J., 2011, 17, 866-872.).
[0088] (a) Synthesis of ethyl 2-tributyltinthieno[3,2-b]thiophene-3-carboxylate (2)
[0089] 1.06g of compound (1) was dissolved in 20mL of dry tetrahydrofuran, and 6.8mL of 1.1M TMPMgCl LiCl was added dropwise thereto under a nitrogen atmosphere at -20°C. After the addition was complete, it was stirred at -20°C for 2 hours, and then Add 2.2mL tributyltin chloride, raise the temperature to room temperature and react for 8-12 hours. After the reaction is completed, pour the reaction liquid into water to quench, and extract twice with petroleum ether; combine the obtained organ...
Embodiment 2
[0115] Embodiment 2: the preparation of acceptor unit BTPA-Br attributed to general formula (II) structure
[0116]
[0117] (a) Synthesis of 2-tributyltinthiophene-3-methyl carboxylate (2)
[0118] 1.42g of compound (1) was dissolved in 40mL of dry tetrahydrofuran, and 13.6mL of 1.1M TMPMgCl LiCl was added dropwise thereto under a nitrogen atmosphere at -20°C. After the addition was complete, it was stirred at -20°C for 2 hours, and then Add 4.4mL tributyltin chloride, raise the temperature to room temperature and react for 8-12 hours. After the reaction is completed, pour the reaction liquid into water to quench, extract twice with petroleum ether, combine the organic phases, dry, filter, and spin dry to obtain crude product, the resulting crude product was passed through Al 2 o 3 Neutral column purification yielded 2.76 g of compound 2 (64%).
[0119] 1 H NMR (CDCl 3 ,400MHz,δ / ppm):7.69(m,1H),7.55(m,1H),3.86(s,3H),1.55(m,6H),1.32(m,6H),1.17(m,6H), 0.89(t,J=7.3Hz,9H...
Embodiment 3
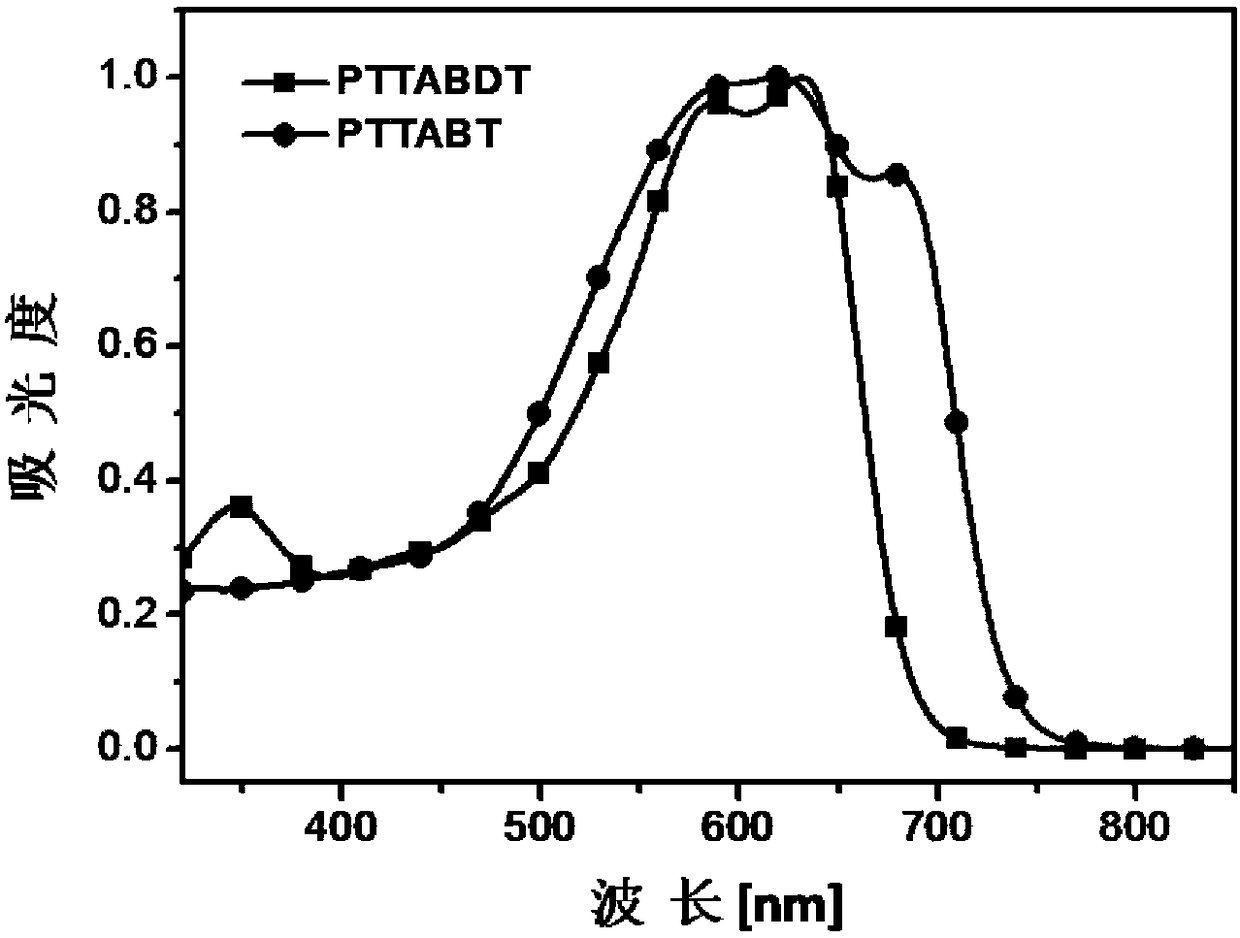
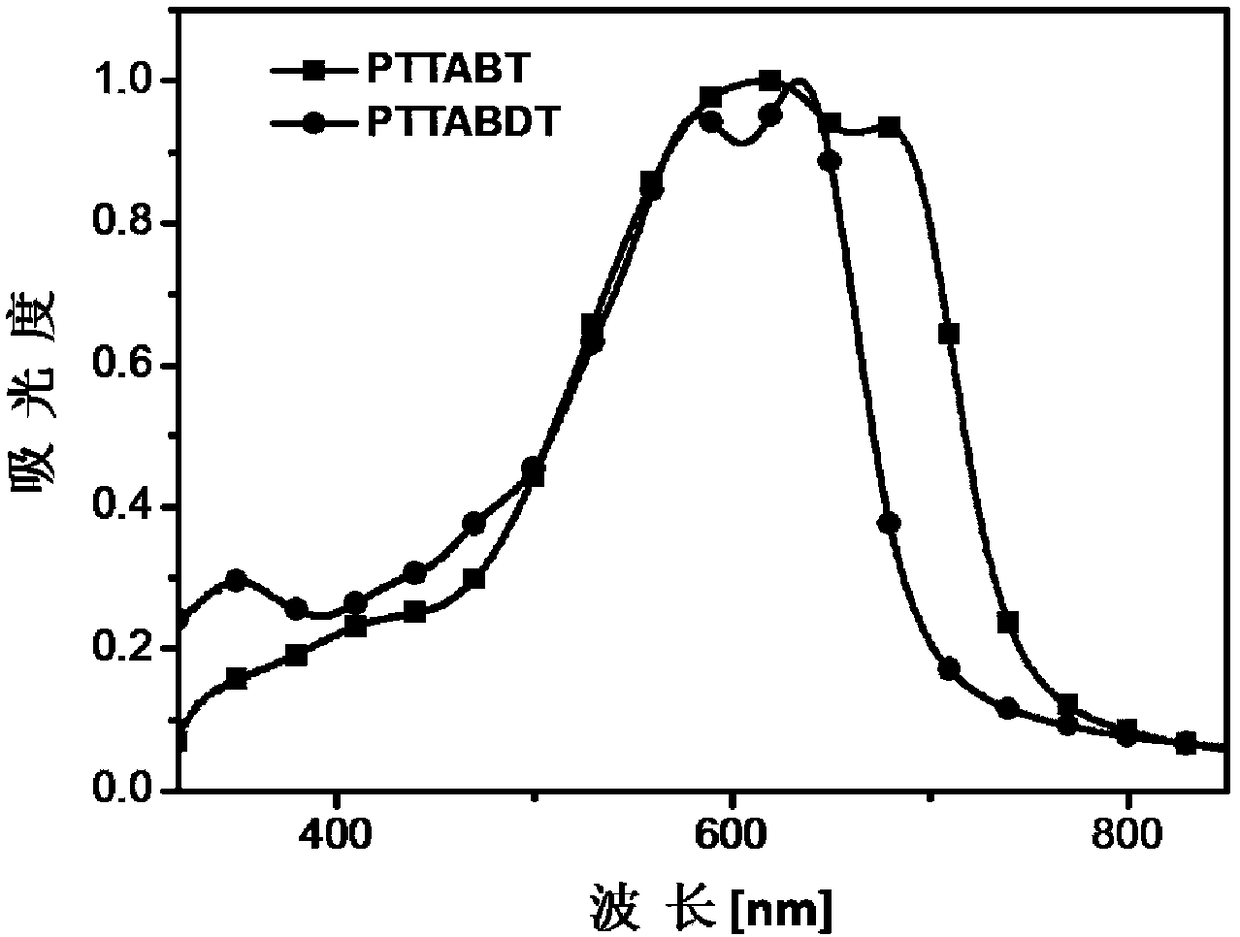
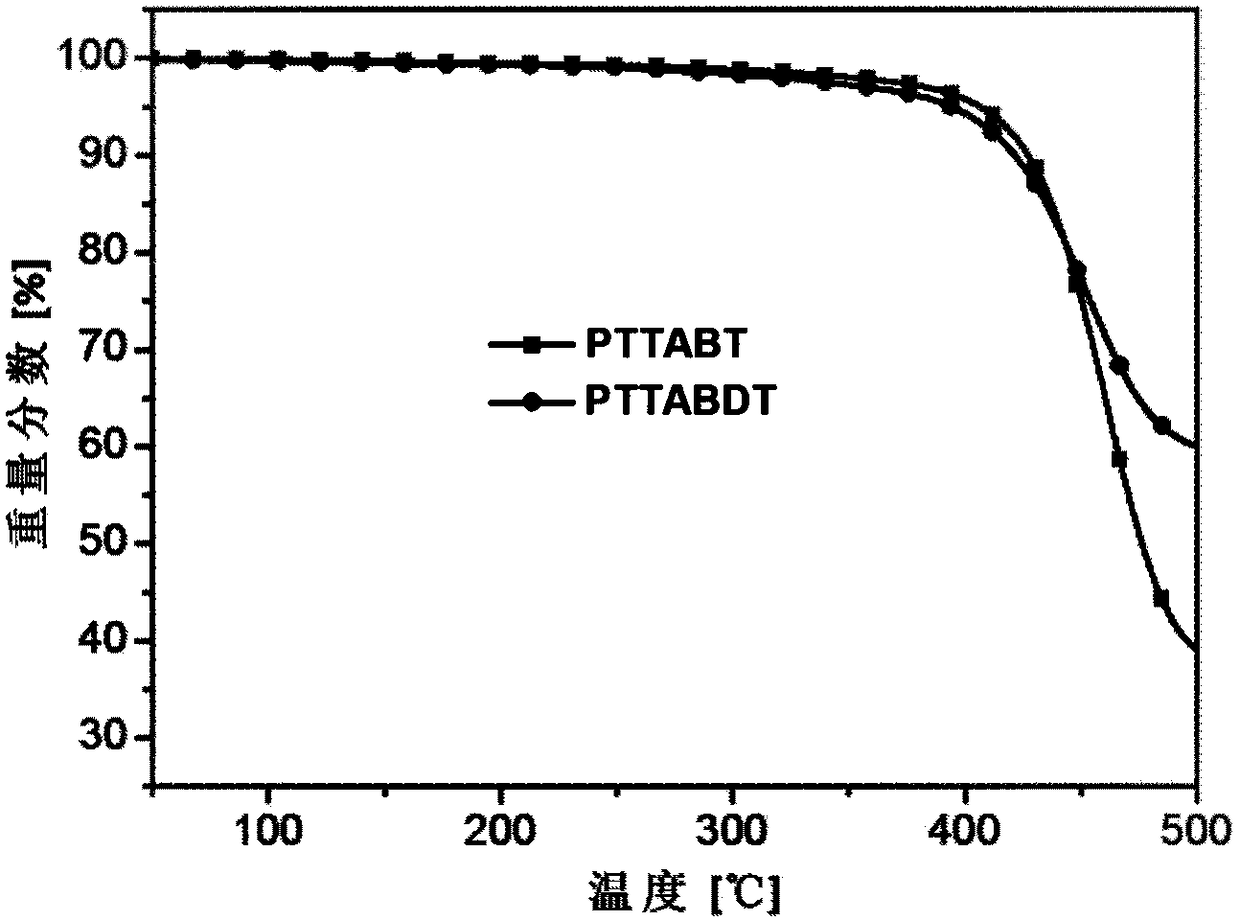
[0137] Copolymerize with TTA-Br prepared in Example 1 and 4,4'-bis(tetradecyl)-5,5'-bis(trimethyltin-yl)-2,2'-bithiophene as monomers Obtain copolymer PTTABT, reaction process is as follows:
[0138]
[0139] Dissolve 78.6 mg of TTA-Br and 88.5 mg of 4,4'-bis(tetradecyl)-5,5'-bis(trimethyltinyl)-2,2'-bithiophene in 20 mL of freshly distilled toluene , add 7mg Pd(PPh 3 ) 4 , and then pass argon for 15 minutes; then under the protection of argon, the solution was heated to reflux for 120 hours, and after the reaction was completed, it was cooled to room temperature, and the reaction solution was dropped into 150mL of methanol, and the crude product was obtained by suction filtration, and was washed with methanol and n-hexane respectively. Alkanes and chloroform were extracted, the chloroform extract was concentrated and dropped into 150 mL of methanol for precipitation again, and 92 mg of a black solid was obtained by suction filtration, with a yield of 77%.
[0140] 1 H ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com