Method for bleaching cotton fabrics with high whiteness effects
A technology of high whiteness, cotton fabrics, applied in the field of high whiteness bleaching of cotton fabrics, can solve the problems of loss of bleaching effect and fiber degradation, and achieve the effect of improving bleaching efficiency, soft hand feeling, and high whiteness requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: The influence of the type of acid agent on the bleaching performance of cotton fabric.
[0022] A kind of bleaching method of cotton fabric, first adopts mineral acid to process scoured cotton fabric, then adopts hydrogen peroxide to bleach, and the steps are as follows:
[0023] Step a. The mineral acid treatment process is: (hydrochloric acid or sulfuric acid) 6mL / L, bath ratio 1:30, 90°C, 6min.
[0024] Step b. The hydrogen peroxide bleaching process is: hydrogen peroxide 6g / L, water glass 5g / L, trisodium phosphate 1g / L, liquor ratio 1:30, pH=10.5, 100°C, 40min.
[0025] Using the above process, test its impact on the bleaching performance of cotton fabrics, as shown in Table 1.
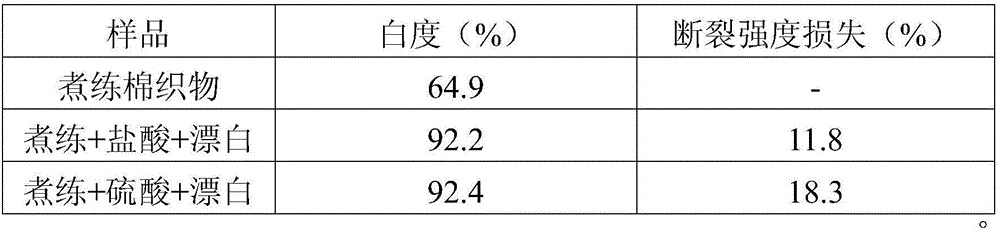
[0026] Table 1. The effect of acid agent types on the bleaching performance of cotton fabrics.
[0027]
[0028] It can be seen from Table 1 that increasing the acid pretreatment before the hydrogen peroxide bleaching process can effectively improve the whiteness of the b...
Embodiment 2
[0029] Example 2: Effect of hydrochloric acid pretreatment on cotton fabric bleaching performance.
[0030] A kind of bleaching method of cotton fabric, first adopts hydrochloric acid to carry out pretreatment to boiled cotton fabric, then adopts hydrogen peroxide to bleach, and the steps are as follows:
[0031] Step a. The hydrochloric acid pretreatment process is: hydrochloric acid 6mL / L, bath ratio 1:30, 90°C, 6min.
[0032] Step b. The hydrogen peroxide bleaching process is: hydrogen peroxide 6g / L, water glass 5g / L, trisodium phosphate 1g / L, liquor ratio 1:30, pH=10.5, 100°C, 40min.
[0033] Using the above process, test its impact on the bleaching performance of cotton fabrics, as shown in Table 2.
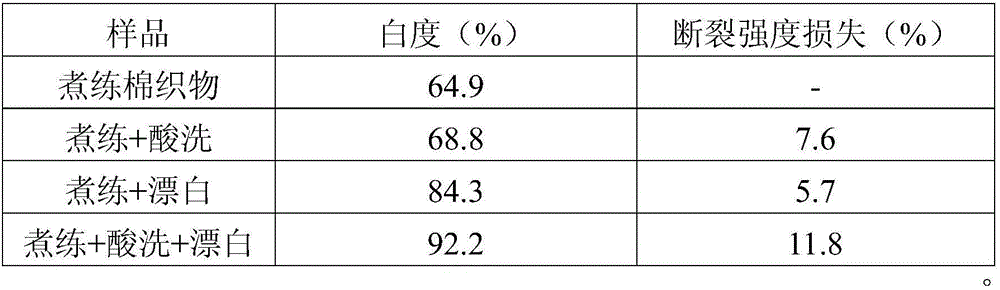
[0034] Table 2. The effect of hydrochloric acid pretreatment on the bleaching performance of cotton fabrics.
[0035]
[0036] It can be seen from Table 2 that adding acid treatment before the hydrogen peroxide bleaching process can effectively improve the whiteness of ...
Embodiment 3
[0037] Embodiment 3: the influence of the amount of hydrochloric acid on the bleaching performance of cotton fabrics.
[0038] A kind of bleaching method of cotton fabric, first adopts hydrochloric acid to process scoured cotton fabric, then adopts hydrogen peroxide to bleach, and the steps are as follows:
[0039] Step a. The hydrochloric acid pretreatment process is: hydrochloric acid 4-8mL / L, bath ratio 1:30, 90°C, 6min.
[0040] Step b. The hydrogen peroxide bleaching process is: hydrogen peroxide 6g / L, water glass 5g / L, trisodium phosphate 1g / L, liquor ratio 1:30, pH=10.5, 100°C, 40min.
[0041] Using the above process, adjust the dosage of hydrochloric acid, and test its effect on the properties of bleached cotton fabrics, as shown in Table 3.
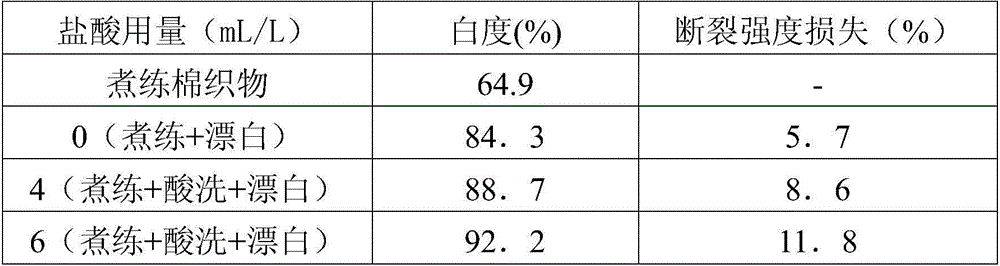
[0042] Table 3. The effect of hydrochloric acid dosage on the properties of bleached cotton fabrics.
[0043]
[0044]
[0045] It can be seen from Table 3 that as the amount of hydrochloric acid increases, the whiteness ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com