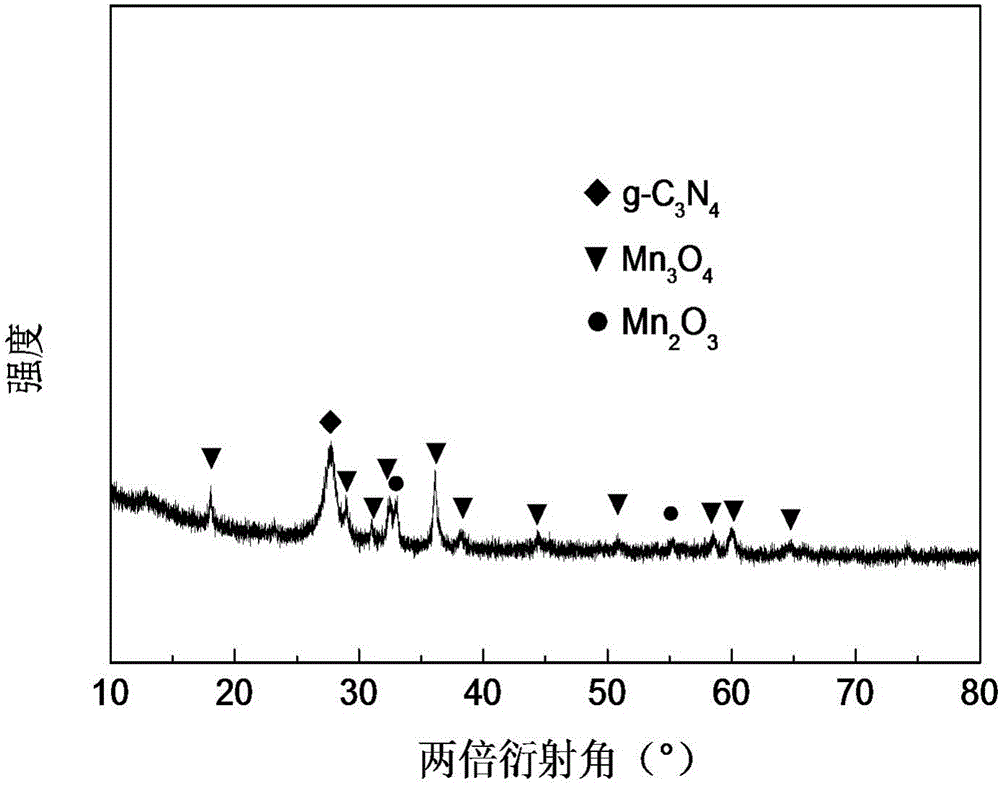
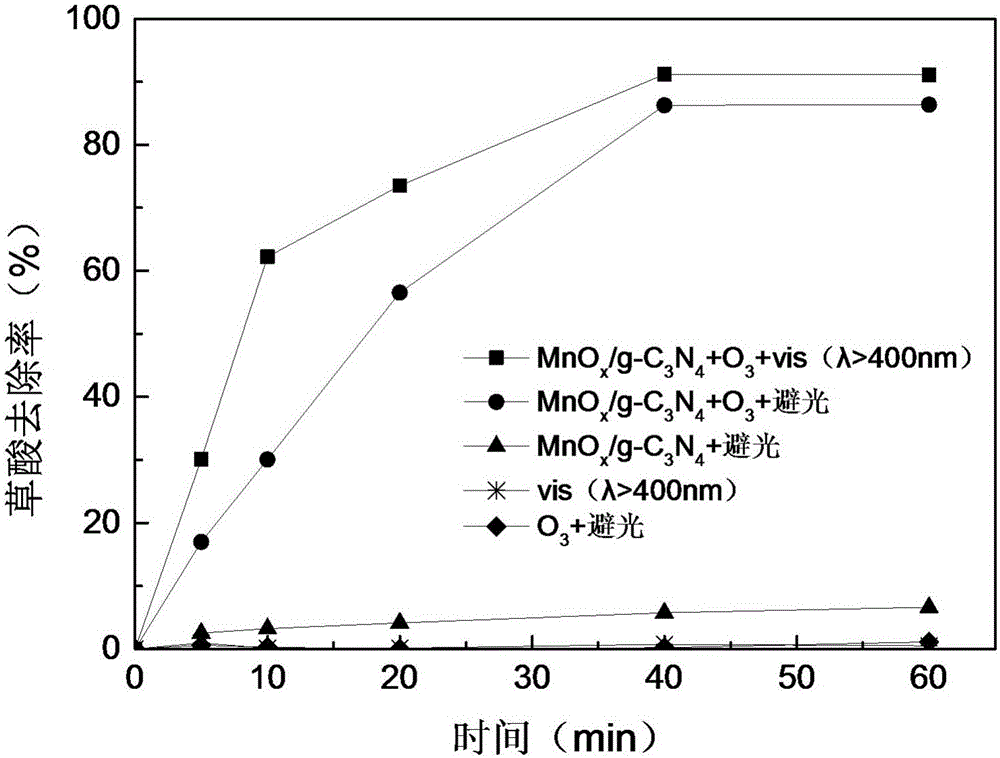
Preparation method and application of visible light and ozone cooperating catalyst for catalytically degrading organic acid
An organic acid catalyst and ozone catalysis technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, oxidized water/sewage treatment, etc., to achieve high catalytic degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of MnO x / g -C 3 N 4 catalyst
[0027] The preparation method of the catalyst for the visible light synergistic ozone catalytic degradation organic acid provided in this embodiment comprises the following steps:
[0028] Step 1, the manganese acetate (III) dihydrate (C 6 h 13 MnO 8 ) and dissolved in 40ml of ultrapure water, after stirring for 5min, 4g of melamine was added to obtain a mixed solution.
[0029] Step 2, heating the mixed solution until the water is evaporated to dryness while stirring to obtain the mixture, and putting the mixture into an oven at a temperature of 90° C. to dry to obtain the dried mixture;
[0030] Step 3: Grinding the dried mixture obtained in Step 2, putting it into a crucible and calcining it in a muffle furnace with a temperature of 550°C (rising temperature: 10°C / min) for 4 hours to obtain a calcined mixture;
[0031] Step 4, after grinding the calcined mixture obtained in step 3, sieve under a sieve of 10...
Embodiment 2
[0034] Example 2 Preparation of MnO x / g -C 3 N 4 catalyst
[0035] The preparation method of the visible light synergistic ozone catalytic degradation organic acid catalyst provided in this embodiment comprises the following steps:
[0036] Step 1, the manganese acetate (III) dihydrate (C 6 h 13 MnO 8 ) and dissolved in 32ml of ultrapure water, after stirring for 10min, 2g of melamine was added to obtain a mixed solution.
[0037] Step 2: heating the mixed solution until the water is evaporated to dryness while stirring to obtain a mixture, and drying the mixture in an oven at a temperature of 80° C. to obtain a dried mixture.
[0038] Step 3: Grinding the dried mixture obtained in Step 2, putting it into a crucible and calcining for 3 hours in a muffle furnace at a temperature of 500°C (rising temperature: 10°C / min) to obtain a calcined mixture.
[0039] Step 4, after grinding the calcined mixture obtained in step 3, sieve under a sieve of 150 mesh (Taylor standard siev...
Embodiment 3
[0040] Example 3 Preparation of MnO x / g -C 3 N 4 catalyst
[0041] The preparation method of the visible light synergistic ozone catalytic degradation organic acid catalyst provided in this embodiment comprises the following steps:
[0042] Step 1, the manganese acetate (III) dihydrate (C 6 h 13 MnO 8 ) and dissolved in 36ml of ultrapure water, after stirring for 8min, 3.2g of melamine was added to obtain a mixed solution.
[0043] Step 2: heating the mixed solution until the water is evaporated to dryness while stirring to obtain a mixture, and drying the mixture in an oven at a temperature of 120° C. to obtain a dried mixture.
[0044] Step 3: Grinding the dried mixture obtained in Step 2, putting it into a crucible and calcining for 5 hours in a muffle furnace at a temperature of 600°C (rising temperature: 10°C / min) to obtain a calcined mixture.
[0045] Step 4, after grinding the calcined mixture obtained in step 3, sieve under a sieve of 200 mesh (Taylor standard ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com