Method for preparing PINO (phthimide-n-oxyl) derivatives through directly coupling NHPI (n-hydroxyphthalimide)and benzyl-containing compounds under non-metal catalysis
A non-metallic catalysis and compound technology, applied in the direction of organic chemistry, can solve the problems of being difficult to apply to industrial production, low safety factor, high cost, etc., and achieve the effect of being conducive to industrial production, low cost and short process
Active Publication Date: 2017-01-04
山东雪地铝业科技有限公司
View PDF4 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
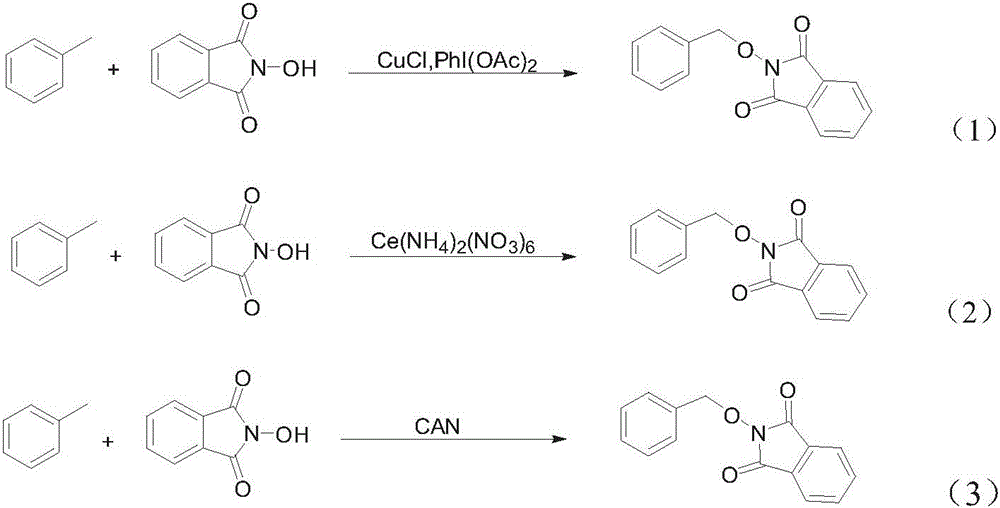
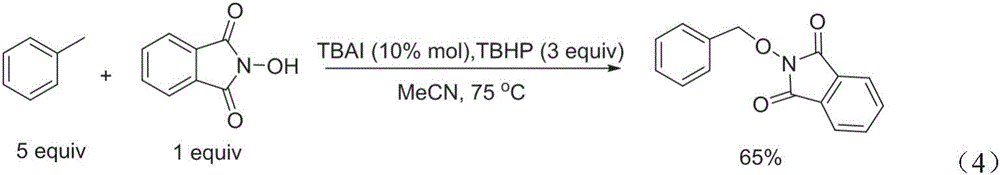
[0008] In view of existing methods for synthesizing PINO derivatives, transition metal catalysts and highly active peroxides are used as oxidizing agents, and the cost is high, the safety factor is low, the yield is low, and it is difficult to be suitable for industrial production. The purpose of the present invention is to provide a A method for synthesizing PINO derivatives with NHPI and benzyl-containing compound raw materials in one step with high yield under mild conditions, the method is low in cost, simple in operation, and meets the requirements of industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
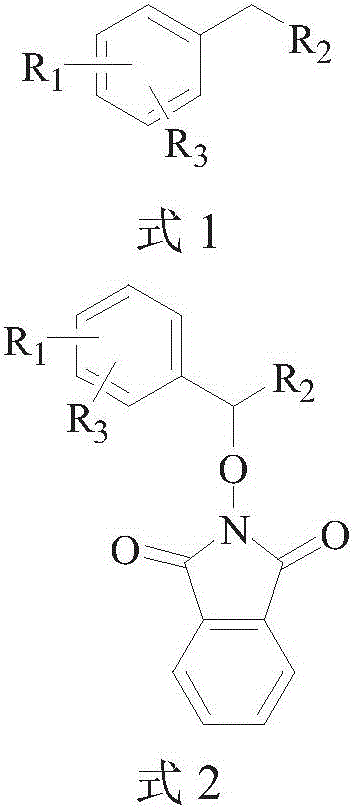
The invention discloses a method for preparing PINO (phthimide-n-oxyl) derivatives through directly coupling NHPI (n-hydroxyphthalimide) and benzyl-containing compounds under non-metal catalysis. According to the method, under the nitrogen protection, the benzyl-containing compounds, N-hydroxyphthalimide and K2S2O8 take a one-pot reaction under the catalysis of tetraalkyl ammonium salts to produce PINO derivatives. By using the method, the next step of reaction is performed under the mild condition; the PINO derivatives are synthesized at a high yield; in addition, the cost is low; the operation is simple; the industrial protection requirements are met.
Description
technical field [0001] The invention relates to a method for preparing PINO derivatives, in particular to a method for preparing PINO derivatives by direct coupling of NHPI and benzyl-containing compounds under non-metal catalysis, and belongs to the field of organic synthesis. Background technique [0002] Free radical chemistry has been a research hotspot for many years because it does play an important role in organic synthesis and industrial applications. It is well known in the art that N-hydroxyphthalimide (NHPI) can generate highly reactive N-oxyphthalimide (PINO) free radicals, which can be used as catalysts or participate in various types of coupling reactions. joint reaction. Some PINO compounds can be easily converted into the corresponding alcohols or hydroxylamines, both of which are very useful intermediates in drug synthesis. In the prior art, several methods for synthesizing PINO compounds have been reported, mainly by using metal salts to catalyze the reac...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D209/48C07D405/12
CPCC07D209/48C07D405/12
Inventor 郭灿城徐静文郭欣
Owner 山东雪地铝业科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com