Novel indolopyrrole compound, preparation method and use thereof
An indolopyrrole and compound technology, which is applied in the field of antibacterial drug preparation, can solve the problems of high synthesis difficulty, poor compound stereoconfiguration selectivity, and lack of visibility, and achieves the effects of simplified synthesis difficulty, good bacteriostatic activity and antibacterial spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
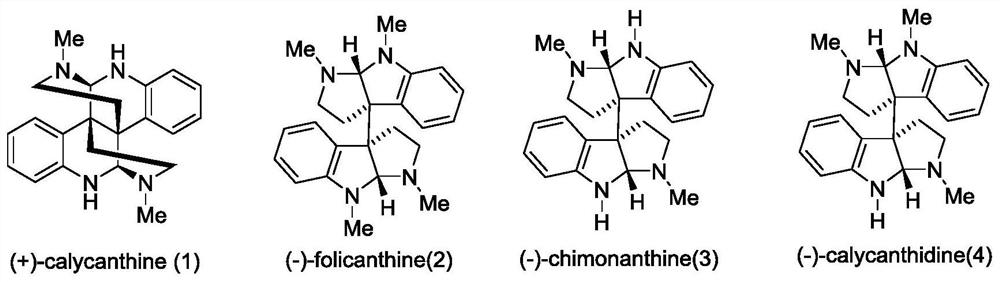
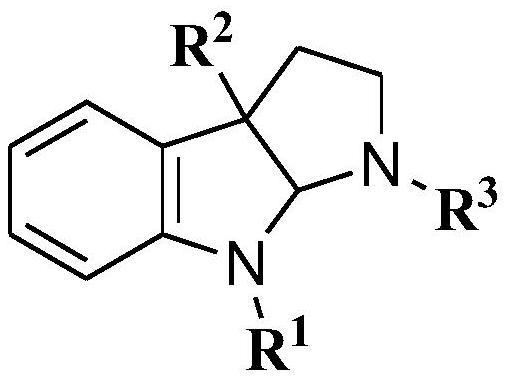
[0034] In order to illustrate the present invention more clearly, the present invention will be further described below in conjunction with preferred embodiments and accompanying drawings. Similar parts in the figures are denoted by the same reference numerals. Those skilled in the art should understand that the content specifically described below is illustrative rather than restrictive, and should not limit the protection scope of the present invention.
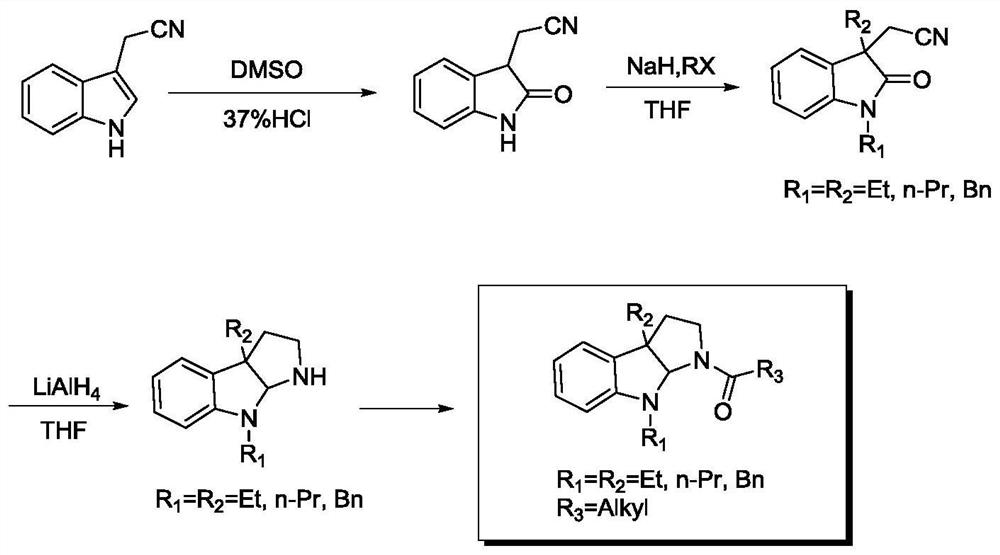
[0035] 1. Synthesis of indolopyrrole compounds
[0036] The synthetic route of the indolopyrrole compound of the present embodiment is shown in formula 1 below:
[0037] (1)Oxindole-3-acetonitrile IAN 1 Synthesis:
[0038]
[0039] Weigh 3.12g (20mmol) of indole-3-acetonitrile and place it in a dry 100mL round-bottomed flask, then add 30mL of DMSO, stir at room temperature for 15min to dissolve completely, place the reaction solution in an ice bath at 0°C, and then Slowly drip 150mL concentrated hydrochloric acid the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com