Bi(tributyltin) trans-butene dibasic acid ester complex as well as preparation method and application thereof
A technology of fumaric acid ester and bis-tributyltin oxide, which can be used in tin organic compounds, drug combinations, anti-tumor drugs, etc., can solve the problems of no anti-cancer activity and high anti-cancer activity, and achieve good anti-cancer The effect of high activity, anticancer activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of two (tributyltin) fumarate complexes:
[0034] Add 0.1181g (1mmol) of fumaric acid, 5968g (1mmol) of bis-tributyltin oxide) and 20mL of solvent methanol in sequence in a 100ml round bottom flask, and react for 8h at a temperature of 50~65°C; cool , filtered, and under the condition of 20~35°C, control the solvent volatilization and crystallization to obtain a white solid, which is bis(tributyltin)fumarate complex. Yield: 65%, melting point: 125-126°C.
[0035] Elemental analysis (C 28 h 56 o 4 sn 2 ): theoretical value: C, 48.45; H, 8.13. Found: C, 48.40; H, 8.16.
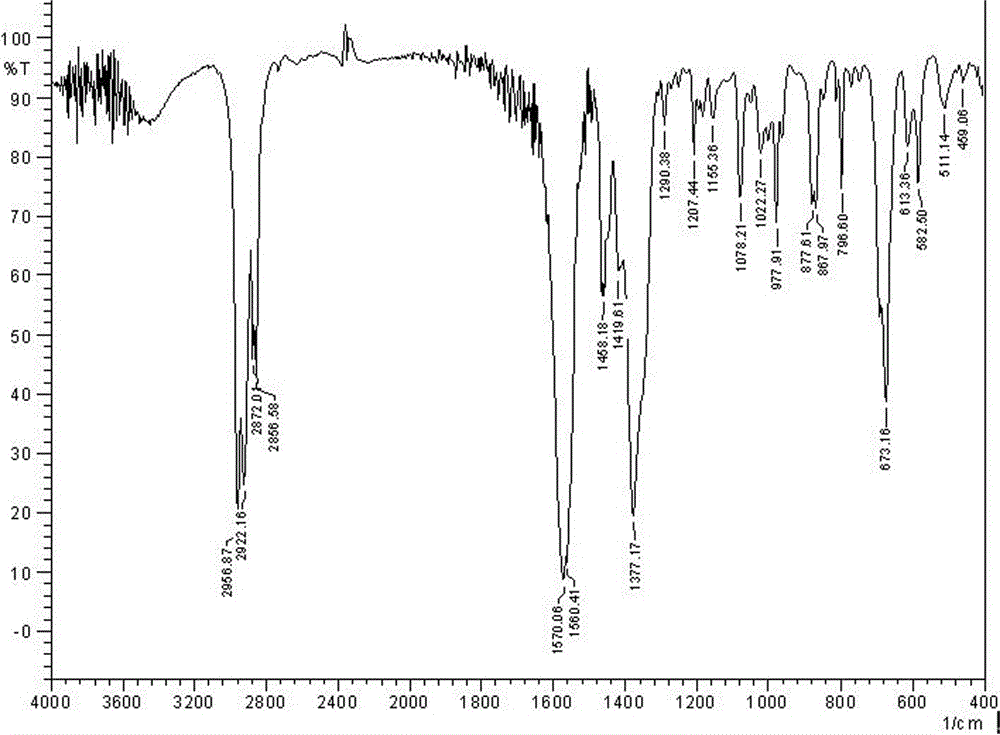
[0036] IR(KBr, cm -1 ): 2957, 2922, 2872, 2857v(C-H), 1570v as (COO - ), 1377v s (COO - ), 613v(Sn-C), 511v(Sn-O).
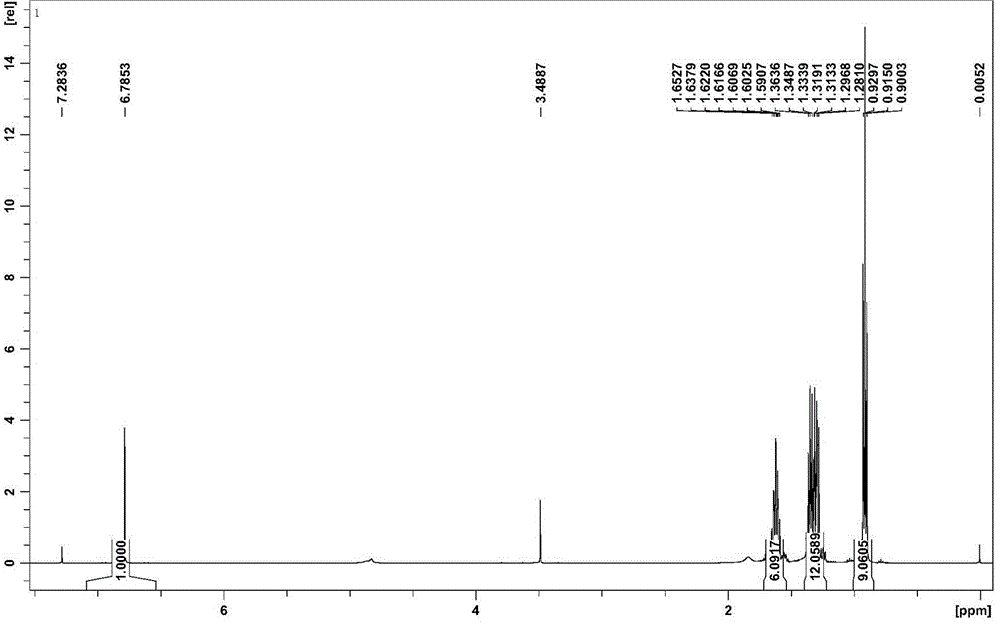
[0037] 1 H NMR (CDCl 3 , 500 MHz), δ(ppm): 6.79(s, 2H, -CH=CH-), 0.90-1.65(m, 54H, Bu-H).
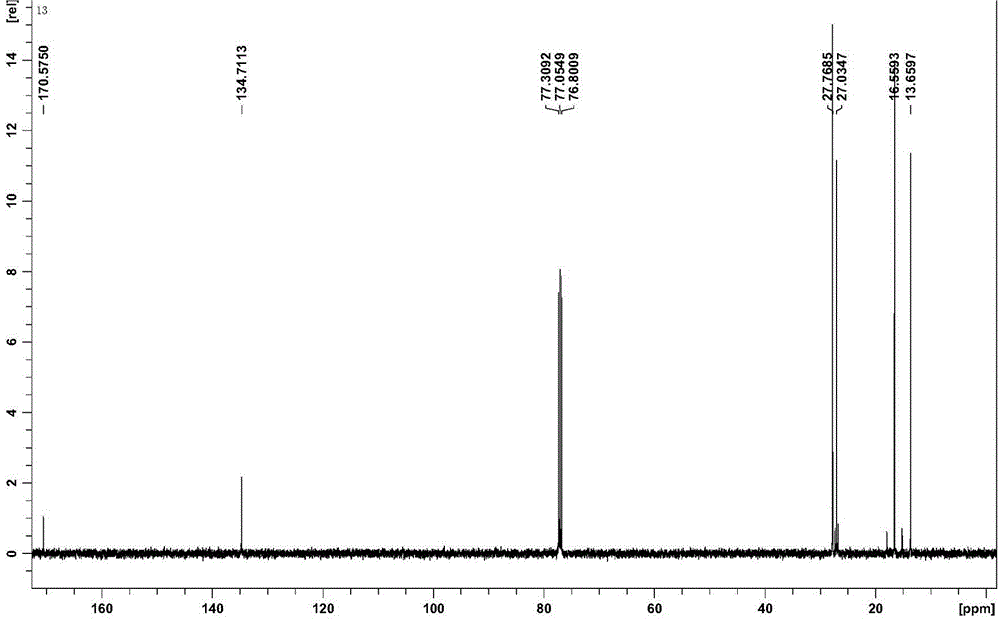
[0038] 13 C NMR (CDCl 3 , 125 MHz), δ (ppm): 170.58 (COO), 134.71 (-CH=CH-), 27.77, 27.03, 16.56, 13.66 (Bu).
[0039] 119 Sn NMR (CDCl 3 ,186 MHz), δ(ppm): 121.73.
Embodiment 2
[0041] Preparation of two (tributyltin) fumarate complexes:
[0042] Add 0.1179g (1mmol) of fumaric acid, 0.6263g (1.05mmol) of bistributyltin oxide, and 37mL of solvent methanol in sequence in a 100ml round bottom flask, and react for 12h at a temperature of 50~65°C; cool , filtered, and under the condition of 20~35°C, control the solvent volatilization and crystallization to obtain a white solid, which is bis(tributyltin)fumarate complex. Yield: 66%, melting point: 125-126°C.
[0043] Elemental analysis (C 28 h 56 o 4 sn 2 ): theoretical value: C, 48.45; H, 8.13. Found: C, 48.40; H, 8.16.
[0044] IR(KBr, cm -1): 2957, 2922, 2872, 2857v(C-H), 1570v as (COO - ), 1377v s (COO - ), 613v(Sn-C), 511v(Sn-O).
[0045] 1 H NMR (CDCl 3 , 500 MHz), δ(ppm): 6.79(s, 2H, -CH=CH-), 0.90-1.65(m, 54H, Bu-H).
[0046] 13 C NMR (CDCl 3 , 125 MHz), δ (ppm): 170.58 (COO), 134.71 (-CH=CH-), 27.77, 27.03, 16.56, 13.66 (Bu).
[0047] 119 Sn NMR (CDCl 3 ,186 MHz), δ(ppm): 121.73...
Embodiment 3
[0049] Preparation of two (tributyltin) fumarate complexes:
[0050] Add fumaric acid 0.2325g (2mmol), bistributyltin oxide 1.1926 (2mmol), and solvent methanol 60mL in sequence in a 100ml round-bottomed flask, and react at a temperature of 50-65°C for 16 hours; cool and filter , Under the condition of 20~35℃, control the solvent volatilization and crystallization to obtain a white solid, which is bis(tributyltin)fumarate complex. Yield: 64%, melting point: 125-126°C.
[0051] Elemental analysis (C 28 h 56 o 4 sn 2 ): theoretical value: C, 48.45; H, 8.13. Found: C, 48.40; H, 8.16.
[0052] IR(KBr, cm -1 ): 2957, 2922, 2872, 2857v(C-H), 1570v as (COO - ), 1377v s (COO - ), 613v(Sn-C), 511v(Sn-O).
[0053] 1 H NMR (CDCl 3 , 500 MHz), δ(ppm): 6.79(s, 2H, -CH=CH-), 0.90-1.65(m, 54H, Bu-H).
[0054] 13 C NMR (CDCl 3 , 125 MHz), δ (ppm): 170.58 (COO), 134.71 (-CH=CH-), 27.77, 27.03, 16.56, 13.66 (Bu).
[0055] 119 Sn NMR (CDCl 3 ,186 MHz), δ(ppm): 121.73.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com