Production method of rare ginsenoside Rh2
A technology of ginsenoside and production method, which is applied in the directions of biochemical equipment and method, botanical equipment and method, enzyme, etc., can solve the problems of high cost, poor stereoselectivity, complex synthesis route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
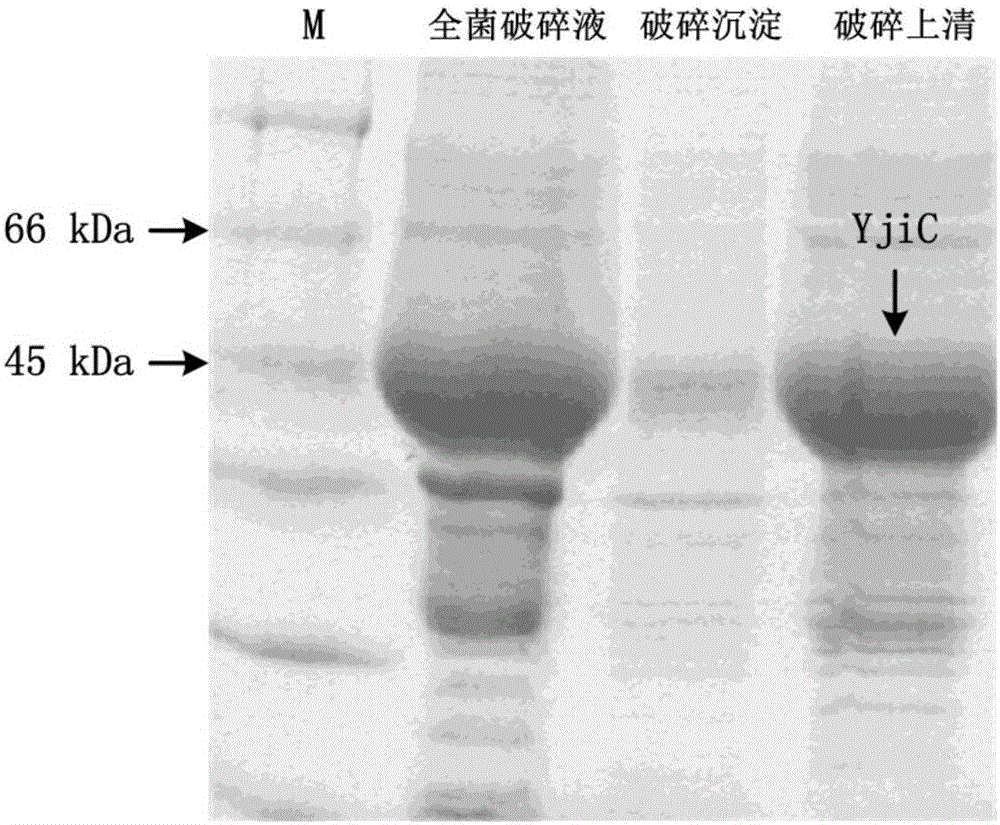
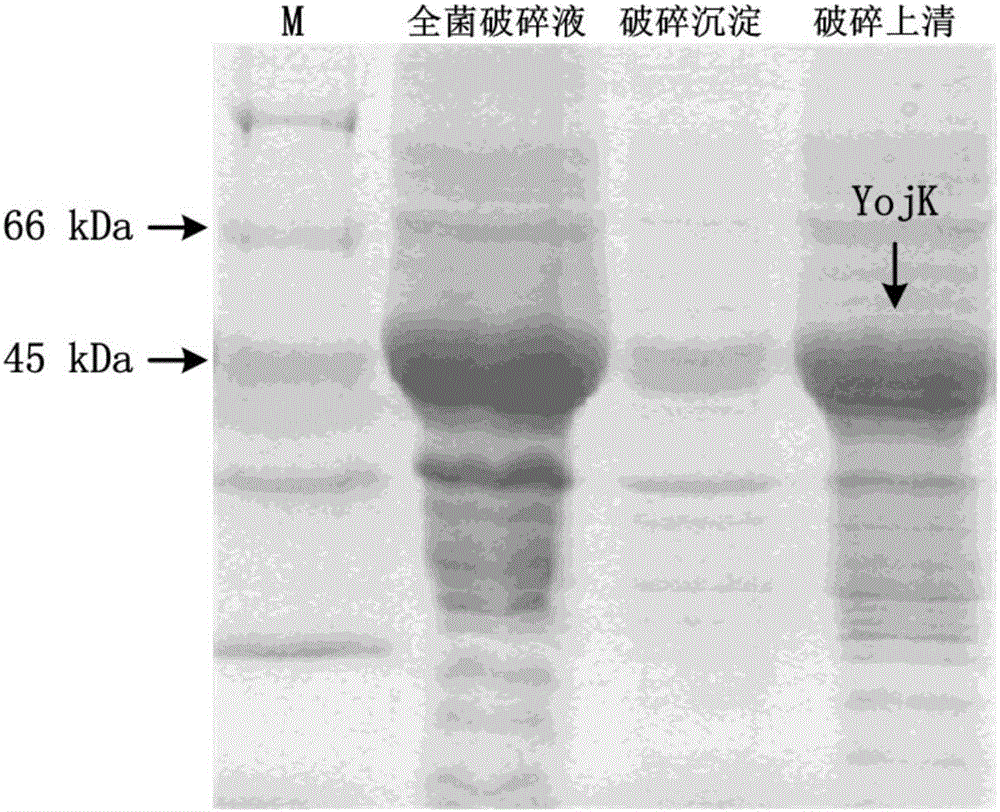
[0048] Example 1 Preparation of Glycosyltransferase
[0049] Bacillus subtilis was extracted using a bacterial genomic DNA extraction kit ( Bacillus subtilis 168), and then using the extracted Bacillus subtilis genomic DNA as a template, according to the primer sequences in Table 1, use the primer pair YjiC-F and YjiC-R and the primer pair YojK-F and YojK-R to amplify respectively The glycosyltransferase genes YjiC and YojK were obtained. The PCR amplification system is: Q5 Buffer (5×) 10 μL, dNTP (2.5 mM) 3 μL, genomic DNA template: 1 μL, primers (10 μM) 2.5 μL each, Q5 high-fidelity polymerase 0.5 μL, complement Double distilled water to 50 μL. The conditions of the PCR reaction were pre-deformation at 98°C for 3 minutes (1 cycle), deformation at 98°C for 10 seconds, annealing for 20 seconds (the annealing temperature was 56°C), extension at 72°C for 1 minute (31 cycles), extension at 72°C for 2 minutes (1 cycle).
[0050] Table 1 Primer sequences
[0051]
[0052]...
Embodiment 2
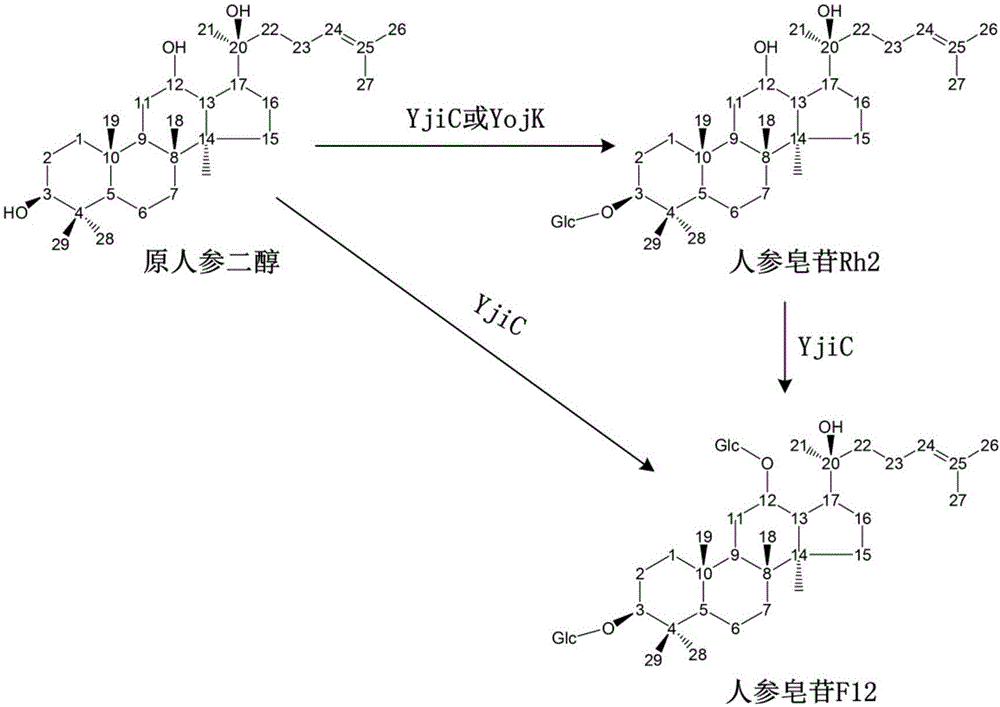
[0055] Example 2 Glycosyltransferase YjiC is used to produce ginsenosides Rh2 and F12
[0056] The glycosylation reaction system includes 25 mM / L Tris-HCl (pH8.0), 1 mM / L protopanaxadiol or ginsenoside Rh2, 3 mM / L UDP-glucose, 10 mM / L MgCl 2. Add glycosyltransferase YjiC crude enzyme solution to the reaction solution at a ratio of 5% (v / v), react at 35°C, 120 rpm, for 4 h.
[0057] After the glycosylation reaction was completed, ethyl acetate was added to extract the glycosylated product, and the extraction was repeated 4 times. Then the glycosylated products extracted for a total of 5 times were combined, concentrated by a rotary evaporator, and then chromatographic methanol was added to redissolve the extracted glycosylated products, and the glycosylated products were detected and quantified by liquid chromatography-mass spectrometry. The liquid chromatograph is Agilent 1260, the chromatographic column is the C18 chromatographic column of Yuexu Technology (Shanghai) Co., L...
Embodiment 3
[0058] Example 3 Glycosyltransferase YojK catalyzes the production of ginsenoside Rh2 from protopanaxadiol
[0059] The glycosylation reaction system includes 25 mM / L Tris-HCl (pH8.0), 1 mM / L protopanaxadiol, 3 mM / L UDP-glucose, 10 mM / L MgCl 2 . Add glycosyltransferase YojK crude enzyme solution to the reaction solution at a ratio of 10% (v / v), react at 35°C, 120 rpm, for 4 h.
[0060] After the glycosylation reaction was completed, ethyl acetate was added to extract the glycosylated product, and the extraction was repeated 4 times. After combining all the extracted products, the extracted products were dried using a rotary evaporator, and then chromatographic methanol was added to dissolve the extracted glycosylated products, and the glycosylated products were detected and quantified by liquid chromatography-mass spectrometry. The liquid chromatograph is Agilent 1260, the chromatographic column is the C18 chromatographic column of Shanghai Yuexu Technology (model: XDB-C18, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com